lowest chair conformation Draw the second chair conformation ring-flip-check this post if not sure. 1 if both methyl groups.
Lowest Chair Conformation, If the number of heavy axial groups becomes smaller when the conformation is changed to 1 C 4 all equatorial groups in 4 C 1 become axial and vice versa then it is likely that the conformation is 1 C 4. C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. Chair Conformations Examples - YouTube.
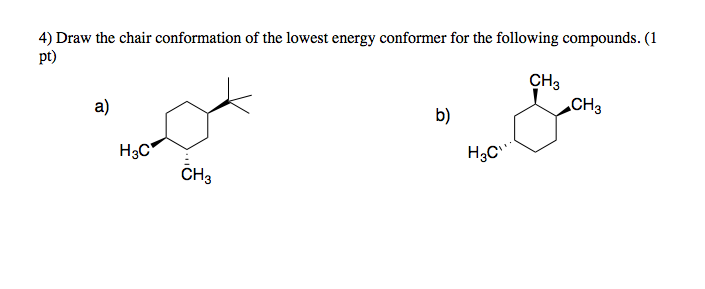 Solved Chair Conformation Of The Lowest Energy Conformer Chegg Com From chegg.com
Solved Chair Conformation Of The Lowest Energy Conformer Chegg Com From chegg.com
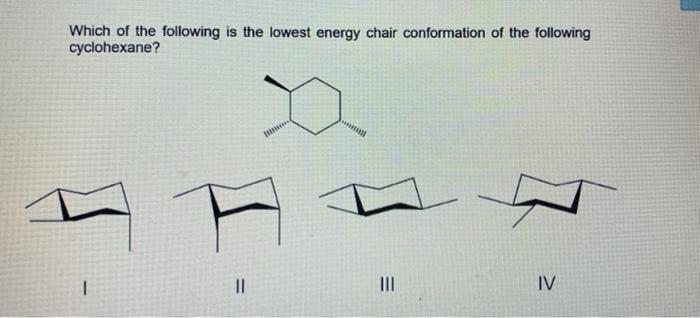
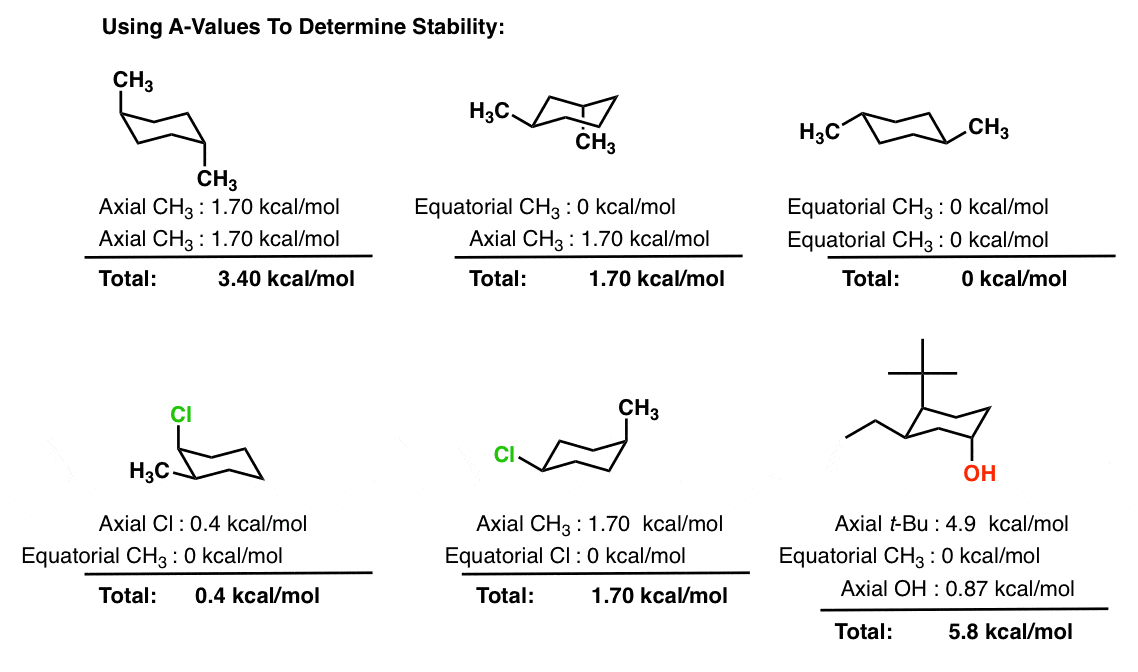
This is true for 1R-33-dichlorocyclohexanol. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1. Steric parameters are tabulated in many organic chemistry textbooks the most common of which is the A-value. Which of the following is the lowest energy chair conformation of the following cyclohexane. And now the stabilities.
Cyclohexane is unique in being the only cyclic hydrocarbon which is.
This means that the chair conformation is the structure that is observable. You can see the chair conformation of cyclohexane in the image to the right. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause. Like in given figure no. If the number of heavy axial groups becomes smaller when the conformation is changed to 1 C 4 all equatorial groups in 4 C 1 become axial and vice versa then it is likely that the conformation is 1 C 4.
Another Article :

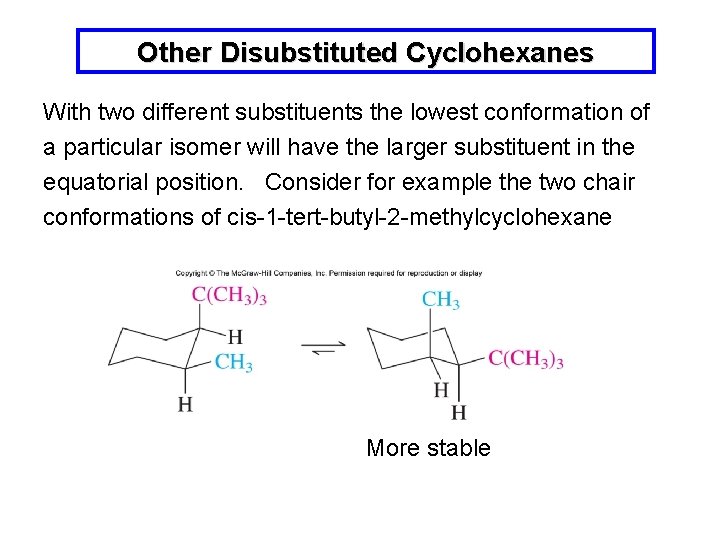
While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations conformations that correspond to local minima on the potential energy surface are specifically called. In this type of conformation there are two. 14 Which of the statements below correctly describes the chair conformations of trans-13-. The lowest energy conformer for a cyclohexane ring with multiple substituents will be the one in which the largest group occupies an equatorial position. If they occupy the axial positions then it will be the highest energy chair conformation because of. D The higher energy chair conformation contains two axial methyl groups. Solution Draw The Lowest Energy Chair Co Organic Chem.
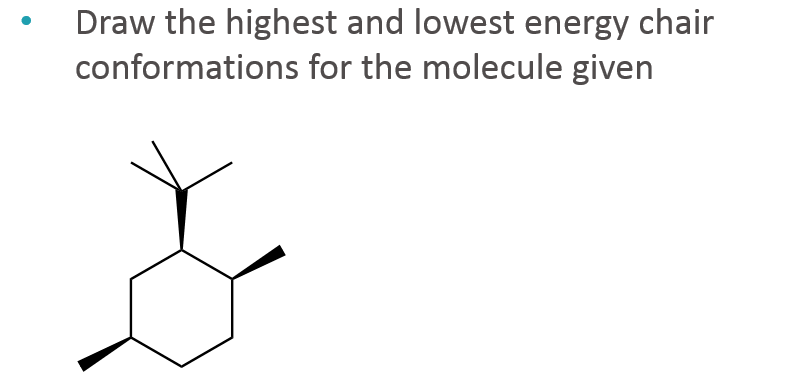
Run a molecular dynamics simulation in order to get a sense of the flexibility of the cyclohexane ring. This is your first axial substituent. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. Solved Draw The Highest And Lowest Energy Chair Chegg Com.
Cyclohexane is unique in being the only cyclic hydrocarbon which is. Steric parameters are tabulated in many organic chemistry textbooks the most common of which is the A-value. In chemistry conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds. A The two chair conformations are equal in energy. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1. Chair Conformations Examples - YouTube. Chemical Change Chapter 2 Dr Suzan A Khayyat.
A The two chair conformations are equal in energy. For each chair conformer add the energy of all the groups on axial position. This is true for 1R-33-dichlorocyclohexanol. And models will always be permitted in examinations. An alternate conformation for a six-membered ring is called the boat. This means that the chair conformation is the structure that is observable. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
The higher energy chair conformation contains two axial methyl groups. D The higher energy chair conformation contains two axial methyl groups. The different conformations are called conformers a blend of the words conformation and isomer. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. If playback doesnt begin shortly try restarting your device. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. Chapter 3 Alkanes And Cycloalkanes Conformations And Cistrans.

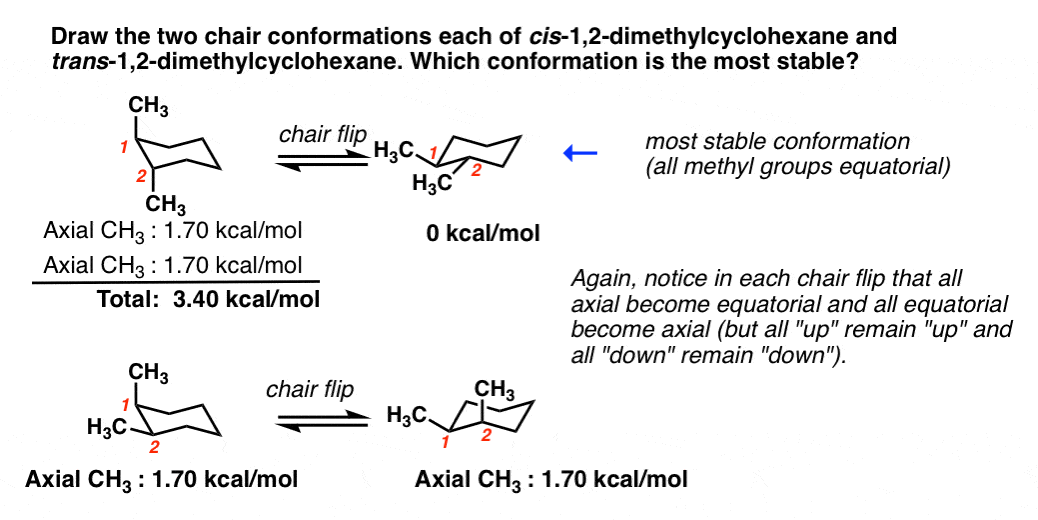
For cis-13-dimethylcyclohexane the lowest energy chair conformation implies that the methyl groups of 1 and 3 carbons of the cyclohexane ring must occupy the equatorial positions. This means that the chair conformation is the structure that is observable. Chair Conformations Examples - YouTube. The lower energy chair conformation contains two axial methyl groups. There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans. The chair conformation is the most stable conformation of cyclohexane. Spectrometer Spectrometers Chemistry Help Teaching Chemistry.
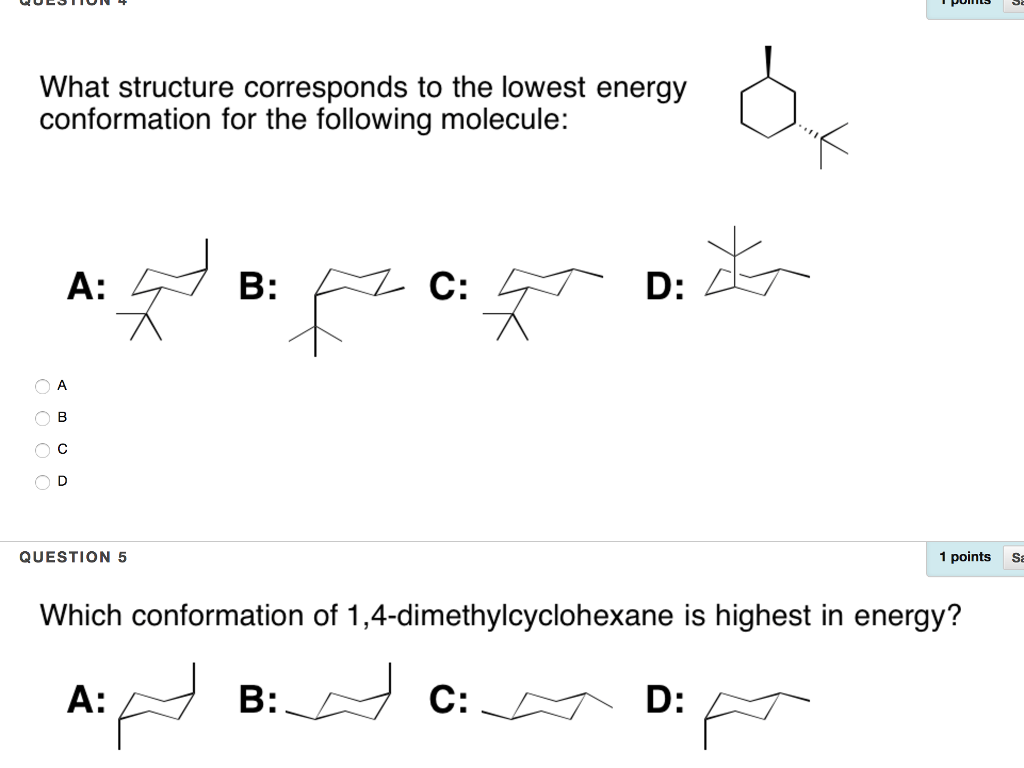
In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. The other conformations are known as the twist and boat forms. If they occupy the axial positions then it will be the highest energy chair conformation because of. The chair conformation is the most stable conformation of cyclohexane. Draw the second chair conformation ring-flip-check this post if not sure. Solved What Structure Corresponds To The Lowest Energy A Chegg Com.
While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations conformations that correspond to local minima on the potential energy surface are specifically called. Draw the second chair conformation ring-flip-check this post if not sure. A The two chair conformations are equal in energy. Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. As we have discussed earlier the chair conformation of cyclohexane is the most stable because it has the lowest strain energy. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. 1 if both methyl groups. If the number of heavy axial groups becomes smaller when the conformation is changed to 1 C 4 all equatorial groups in 4 C 1 become axial and vice versa then it is likely that the conformation is 1 C 4. There is no substitute for the use of models. Lowest energy form is the most important. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. Solved Which Of The Following Is The Lowest Energy Chair Chegg Com.
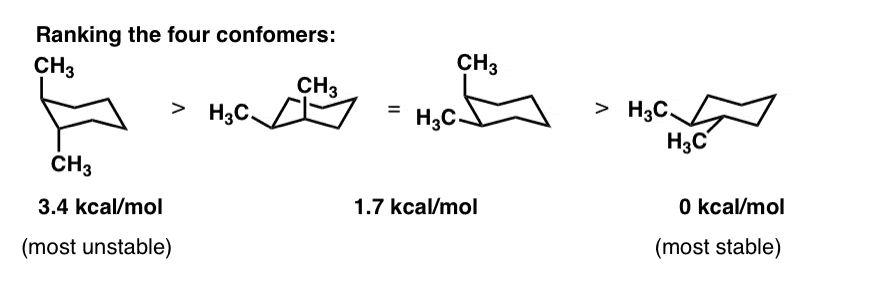
The conformation with the lowest strain energy is the chair conformation. For cis-13-dimethylcyclohexane the lowest energy chair conformation implies that the methyl groups of 1 and 3 carbons of the cyclohexane ring must occupy the equatorial positions. This means that the chair conformation is the structure that is observable. And models will always be permitted in examinations. I am asked to draw the most stable chair conformation of compound 1. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
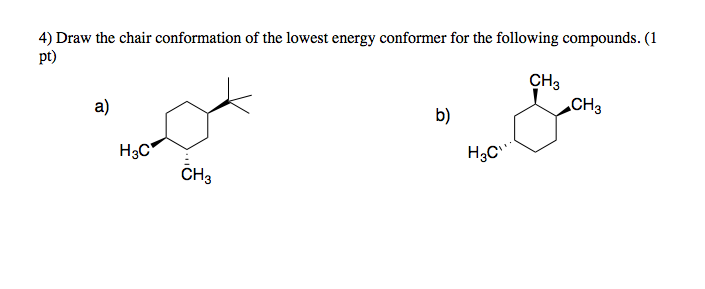
This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. Cyclohexanes undergo fairly facile conformational exchange where axial substituents are exchanged for equatorial substituents and vice versa. The higher energy chair conformation contains two axial methyl groups. Lowest energy form is the most important. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Solved Chair Conformation Of The Lowest Energy Conformer Chegg Com.
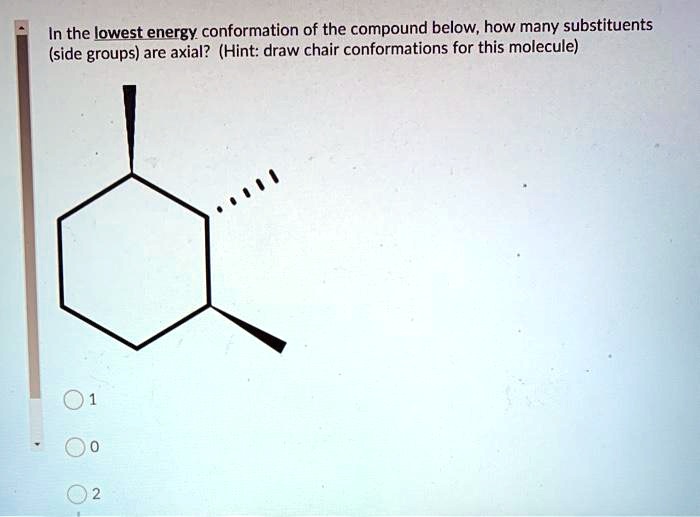
There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans. The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. For each chair conformer add the energy of all the groups on axial position. 10 Jul2017 Tutor. Zxiiojjrv87j3m.
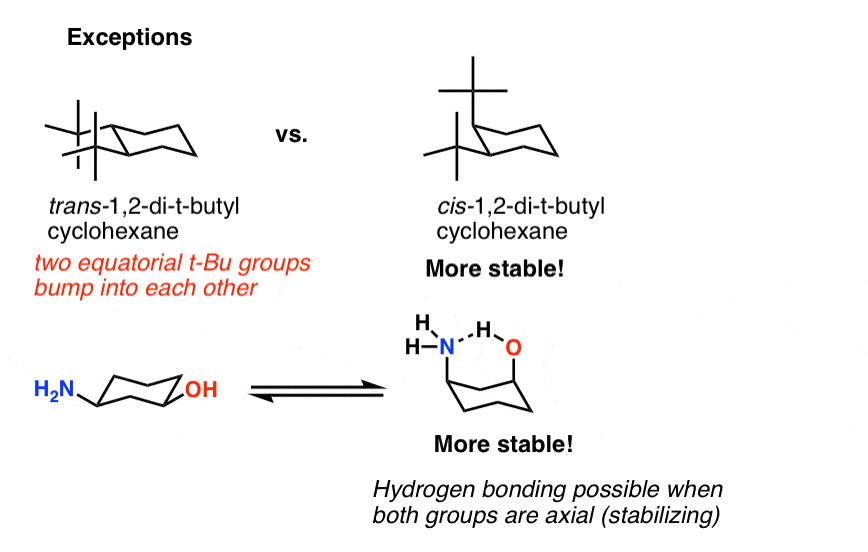
While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations conformations that correspond to local minima on the potential energy surface are specifically called. This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings. The lowest energy conformer for a cyclohexane ring with multiple substituents will be the one in which the largest group occupies an equatorial position. C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. Run a molecular dynamics simulation in order to get a sense of the flexibility of the cyclohexane ring. E The lower energy chair conformation contains two axial methyl groups. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.

C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. The chair conformation is the most stable conformer. In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. You can see the chair conformation of cyclohexane in the image to the right. This means that the chair conformation is the structure that is observable. Lowest energy form is the most important. Rules For Naming Alkanes Organicchemistry Orgo Ochem Mcat Chemistry Lessons Organic Chemistry Organic Chemistry Study.

The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. I am asked to draw the most stable chair conformation of compound 1. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. E The lower energy chair conformation contains two axial methyl groups. Cyclohexanes undergo fairly facile conformational exchange where axial substituents are exchanged for equatorial substituents and vice versa. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Three Of The Lowest Minimum Energy Conformations Of Cyclooctane Download Scientific Diagram.