chair conformations in equilibrium Each carbon atom on a cyclohexane ring has one axial and one equator. Draw the corresponding planar overhead representation using wedge-and-dash bonds to indicate the substituent positions.
Chair Conformations In Equilibrium, Thus at 50C 938 of equatorial conformation and 62 of axial conformation exists in equilibrium. The proton NMR spectrum of cyclohexane is a singlet at room temperature. At 100C 913 of equatorial conformation and 87 of axial conformation exists in equilibrium.
 Group Theory Organic Chemistry Chemistry Classroom Chemistry Lessons From pinterest.com
Group Theory Organic Chemistry Chemistry Classroom Chemistry Lessons From pinterest.com
In one chair form the dihedral angle of the chain of carbon atoms 1-2-3-4 is positive whereas that of the chain 1-6-5-4 is negative but in the other chair form the situation is the opposite. 72 CONFORMATIONS OF CYCLOHEXANE 269 exactly the same contribution to its heat of formation -207 kJ mol_1 or -495 kcal mol_1. We have a myth. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. So far we have not described in detail the rotational journey of the cyclohexane and what is the final result.
If your first chair has the upper line on the right draw the second chair with the upper line left.
And now the stabilities. 5 in favor of the one where the methyl group is equatorial. The preference for these low energy conformations is dictated by the relative orientations of the hydroxyl groups. Chair conformation equilibrium The various conformations of cyclohexane are m rapid equilibrium with one another but at any moment almost all of the molecules exist m the chair conformation Not more than one or two molecules per thousand are present m the skew boat confer matron Thus the discussion of cyclohexane conformational analysis that follows focuses exclusively on the chair conformation. In the case of D-glucopyranoses only the 4C1 conformation is of importance whereas the 1C4 conformation dominates in α-D-idopyranose.
Another Article :

The proton NMR spectrum of cyclohexane is a singlet at room temperature. Cyclohexane is the most widely occurring ring in compounds of natural origin. This is true for 1R-33-dichlorocyclohexanol. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers. 5 in favor of the one where the methyl group is equatorial. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. 6 1b Organic Chemistry Biochemistry Various Organic Functional Group Cset Study Gu Functional Groups Organic Chemistry Functional Group Organic Chemistry.

Draw the second chair conformation ring-flip-check this post if not sure. Now for the fun part determine the new location for the up-tip carbon by pulling down your up-tip or raising up your down-tip Number your new chair and play match the numbers. So here we have two different substitue INTs. 50 mixture of the conformationsIt is shifted to the more stable chair conformation. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers. This is true for 1R-33-dichlorocyclohexanol. Cyclohexane Chair Conformation Stability Which One Is Lower Energy Science Notes Chemistry Notes Organic Chemistry Notes.

Chair conformation equilibrium The various conformations of cyclohexane are m rapid equilibrium with one another but at any moment almost all of the molecules exist m the chair conformation Not more than one or two molecules per thousand are present m the skew boat confer matron Thus the discussion of cyclohexane conformational analysis that follows focuses exclusively on the chair conformation. In the illustration above the two chair conformations are in equilibrium. 50 mixture of the conformationsIt is shifted to the more stable chair conformation. 76 For each of the following compounds draw the two chair conformations that are in equilibrium. So here we have two different substitue INTs. This is true for 1R-33-dichlorocyclohexanol. The Mechanism Of The Wittig Reaction Wittig Reaction Organic Chemistry Chemistry.

In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon atoms 24 and 6 are below the plane. This means that cyclohexane has the same stability as a typical unbranched alkane. For each chair conformer add the energy of all the groups on axial position. The proton NMR spectrum of cyclohexane is a singlet at room temperature. So Ive got my two confirmations drawn out below. At room temperature the two chair conformations rapidly equilibrate. Sodium Borohydride Nabh4 Reduction Reaction Mechanism Organic Chemistry Study Organic Chemistry Reactions Organic Chemistry.

At room temperature methylcyclohexane exists as a rapid equilibrium between the two chair forms and many other intermediate conformations but the equilibrium constant K eq favors the conformation where the methyl group is equatorial. For each chair conformer add the energy of all the groups on axial position. The preference for these low energy conformations is dictated by the relative orientations of the hydroxyl groups. Cyclohexane exist as two chair conformations in rapid equilibrium at room temperature. Thus at 50C 938 of equatorial conformation and 62 of axial conformation exists in equilibrium. Now for the fun part determine the new location for the up-tip carbon by pulling down your up-tip or raising up your down-tip Number your new chair and play match the numbers. Group Theory Organic Chemistry Chemistry Classroom Chemistry Lessons.

Thus at equilibrium and 25C 95 of equatorial-conformation and 5 of axial-conformation exists. This means that cyclohexane has the same stability as a typical unbranched alkane. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. Thus at equilibrium and 25C 95 of equatorial-conformation and 5 of axial-conformation exists. A cis-13-dimethylcyclohexane b trans-1-ethyl-4-isopropylcyclohexane 77 For each of the compounds in Problem 76 draw a boat conformation. Below the figure shows the chair conformations of cis-1 3-dimethylcyclohexane. Translation On The Ribosome Protein Synthesis Central Dogma Amino Acids.

And now the stabilities. The proton NMR spectrum of cyclohexane is a singlet at room temperature. Thus at 50C 938 of equatorial conformation and 62 of axial conformation exists in equilibrium. Cyclohexane is the most widely occurring ring in compounds of natural origin. This means that cyclohexane has the same stability as a typical unbranched alkane. Now for the fun part determine the new location for the up-tip carbon by pulling down your up-tip or raising up your down-tip Number your new chair and play match the numbers. Conformations Of Cyclohexanes Conformations Of Cyclohexanes Mcat Mcatprep Mcatstudying Organicchemistry Organicchem Organik Kimya Biyokimya Kimya.

Chair conformations in equilibrium You should be able to quickly draw cyclohexane rings in which the axial and equatorial ties are easily identifiable and distinguishable. A boat conformation and a chair conformation. In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon atoms 24 and 6 are below the plane. The ratio of two chair conformations in the following mixture is 95. We have a myth. The proton NMR spectrum of cyclohexane is a singlet at room temperature. Pin On Mcat.

So far we have not described in detail the rotational journey of the cyclohexane and what is the final result. This is a multistep process so here Im going to walk you through it from scratch. Thus at 50C 938 of equatorial conformation and 62 of axial conformation exists in equilibrium. The proton NMR spectrum of cyclohexane is a singlet at room temperature. In the illustration above the two chair conformations are in equilibrium. Chair conformation equilibrium The various conformations of cyclohexane are m rapid equilibrium with one another but at any moment almost all of the molecules exist m the chair conformation Not more than one or two molecules per thousand are present m the skew boat confer matron Thus the discussion of cyclohexane conformational analysis that follows focuses exclusively on the chair conformation. Organic Chemistry Nomenclature Organic Chemistry Nomenclature Chemistry Organic Che Organic Chemistry Cheat Sheet Organic Chemistry Notes Chemistry Notes.

For trans-1-ethyl-4-isopropylcyclohexane which two chair conformations are in equilibrium. For cis-1 3-dimethylcyclohexane which two chair conformations are in equilibrium. Below the figure shows the chair conformations of cis-1 3-dimethylcyclohexane. A trisubstituted cyclohexane compound is given below in its chair conformation. Cases occur as in β-D-arabinopyranose where both chair conformations are in equilibrium. Recall that for cis or trans is based on whether the groups are facing the same face top or bottom of the ring. Kinetics Rate Laws Science Chemistry Chemistry Education Chemistry.

In the illustration above the two chair conformations are in equilibrium. A boat conformation and a chair conformation. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal gas constant 1. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. 5 in favor of the one where the methyl group is equatorial. If your first chair has the upper line on the right draw the second chair with the upper line left. Organic Chemistry I Study Guides Organic Chemistry Organic Chemistry Study Chemistry Basics.
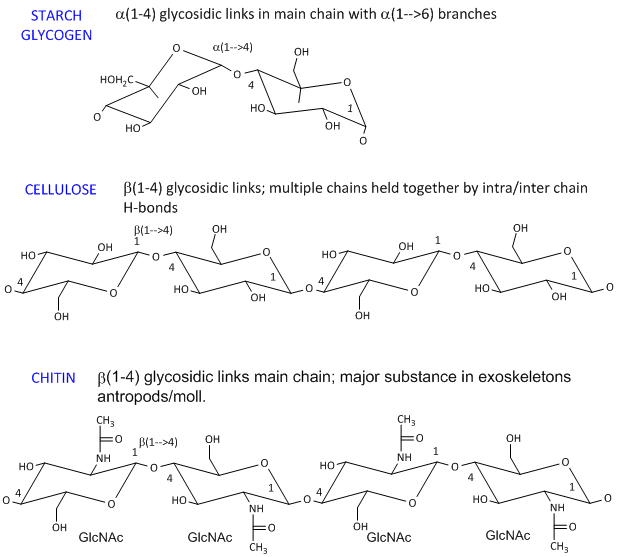
Recall that for cis or trans is based on whether the groups are facing the same face top or bottom of the ring. If your first chair has the upper line on the right draw the second chair with the upper line left. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. The preference for these low energy conformations is dictated by the relative orientations of the hydroxyl groups. This means that cyclohexane has the same stability as a typical unbranched alkane. For trans-1-ethyl-4-isopropylcyclohexane which two chair conformations are in equilibrium. Macromolecules Biochemistry Energy Storage.

In real sense the cyclohexanes adopts two conformations. The boat conformation feels some what greater sterric hindrance so it is less often found while the chair conformation is very stable hence it is favored. A boat conformation and a chair. In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon atoms 24 and 6 are below the plane. Thats what weve drawn out is the chair confirmations for the Trans one type beautiful three missile Cyclo hexane. Recall that for cis or trans is based on whether the groups are facing the same face top or bottom of the ring. Pin On Chemistry.

And now the stabilities. Each carbon atom on a cyclohexane ring has one axial and one equator. If your first chair has the upper line on the right draw the second chair with the upper line left. This is true for 1R-33-dichlorocyclohexanol. Draw the corresponding planar overhead representation using wedge-and-dash bonds to indicate the substituent positions. This means that cyclohexane has the same stability as a typical unbranched alkane. Steric Repulsion In Conformers Axial And Equatorial Covalent Bonding Teaching Chemistry Study Chemistry.

For cis-13-dimethylcyclohexane which two chair conformations are in equilibrium. This is a multistep process so here Im going to walk you through it from scratch. Below the figure shows the chair conformations of cis-1 3-dimethylcyclohexane. This is true for 1R-33-dichlorocyclohexanol. The ratio of two chair conformations in the following mixture is 95. Chair conformations in equilibrium You should be able to quickly draw cyclohexane rings in which the axial and equatorial ties are easily identifiable and distinguishable. Video How To Draw Cyclohexane Chair Conformations And Ring Flips Organicchemistry Chemistry Education Organic Chemistry Online Tutoring.










