chair conformation process Two 1 H NMR signals should be observed in principle corresponding to axial and equatorial protons. And now the stabilities.
Chair Conformation Process, Step 4 illustrates the addition of. Draw the second chair conformation ring-flip-check this post if not sure. Show this interconversion by drawing the two chair conformations and putting a reversible arrow.
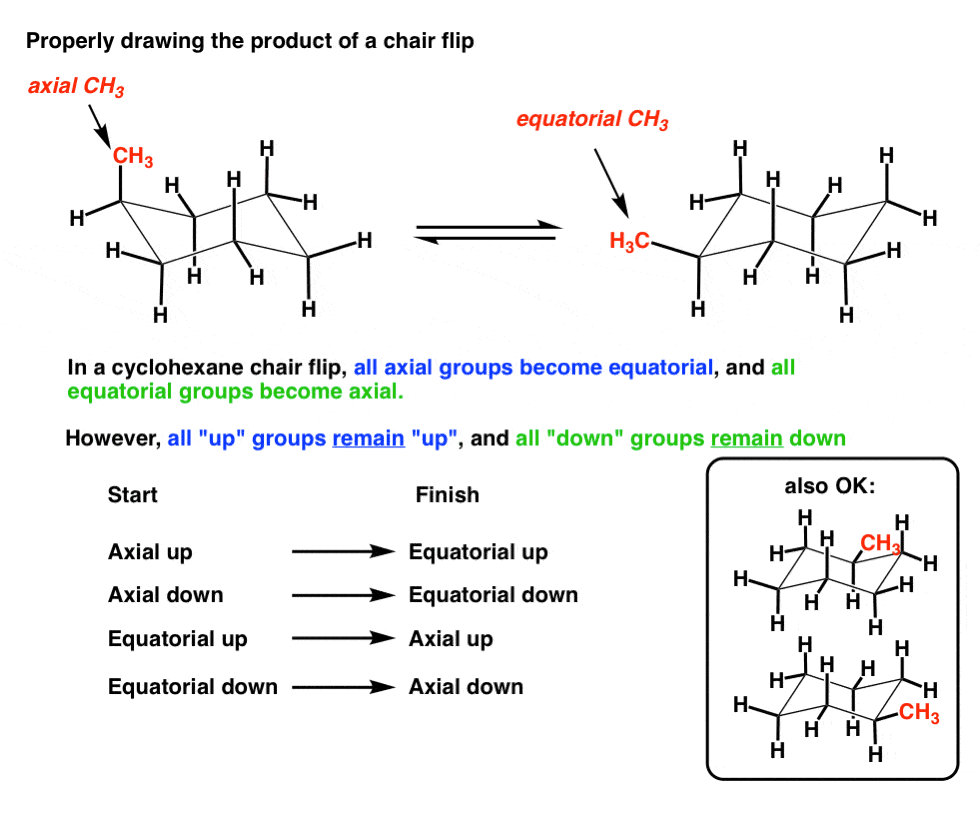 The Cyclohexane Chair Flip Master Organic Chemistry From masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry From masterorganicchemistry.com
In the ring-flipping process C. Half-chair boat half-chair to get to the other chair conformation. Number the ring and draw any chair conformation of the compound. Use the guidelines below place substituents in the proper axialequatorial orientation. In the ring-flipping process the chair goes through the following conformations.
CONFORMATIONAL ANALYSIS OF MONOSUBSTITUTED CYCLOHEXANES The most stable conformation is a chair with the.
But notice what has happened to the hydrogens. Two 1 H NMR signals should be observed in principle corresponding to axial and equatorial protons. But notice what has happened to the hydrogens. The preferred conformation of the tetrahydropyran ring is the chair. In step 1 two circles are drawn side-by-side.
Another Article :

Draw the second chair conformation ring-flip-check this post if not sure. The chair conformation of cyclohexane is not rigid. If a particular axial hydrogen atom points up the axial hydrogen atoms on the two adjacent carbon atoms point down. The chair is the strongly preferred conformation. The preferred conformation of the tetrahydropyran ring is the chair. Six point up or down with respect to the average plane of the ring of carbon atoms. The Cyclohexane Chair Flip Master Organic Chemistry.
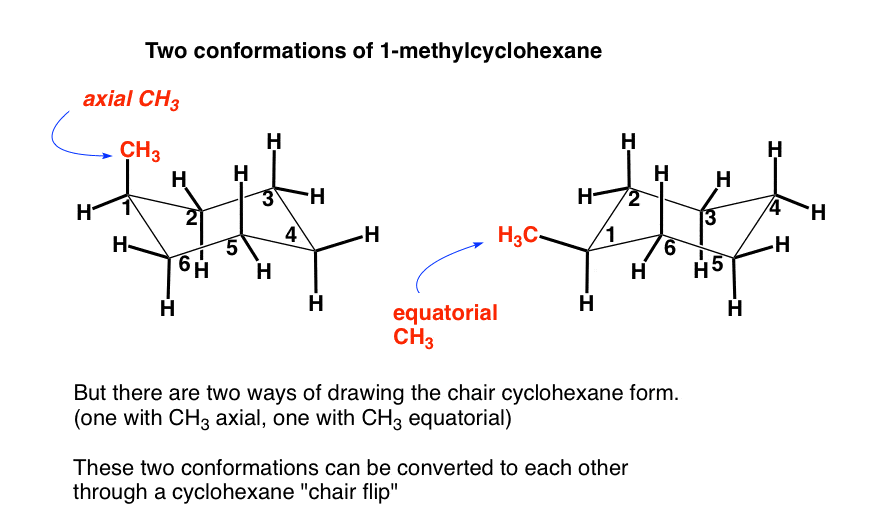
The chair conformation is free of torsional strain as well. In this process the equa-torial hydrogens have become axial and the axial hydrogens have become equatorial. Moreover the hydrogen atoms at opposite corners. Upon ring flipping the axial and equatorial bonds interchange their positions. When viewed along any carboncarbon bond viewing the structure from an end Fig2 the bonds are seen to be perfectly staggered. The process of ring flipping takes cyclohexane through a conformation called the twist chair or the half chair form and this form is 108kcal less stable than the chair conformer. The Cyclohexane Chair Flip Master Organic Chemistry.
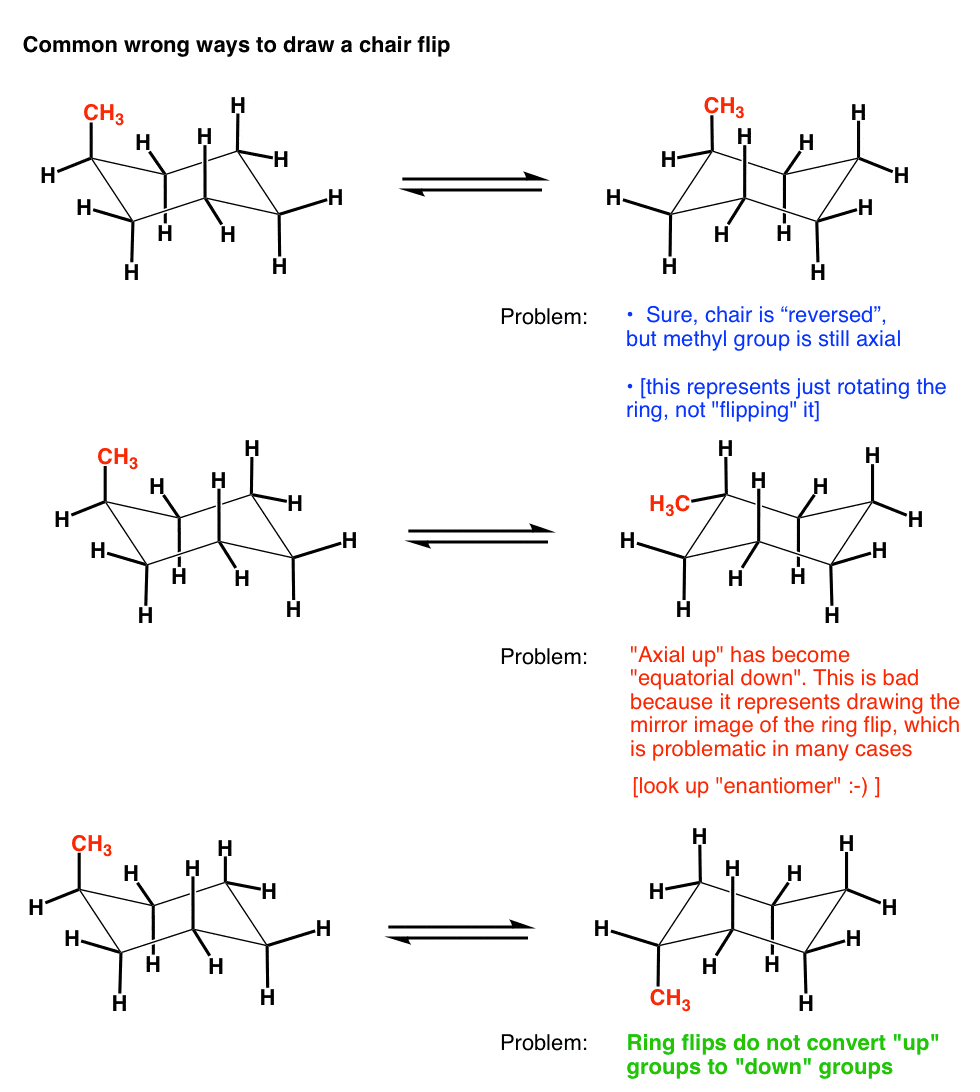
In the ring-flipping process C. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. And now the stabilities. Moreover the hydrogen atoms at opposite corners. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. CONFORMATIONAL ANALYSIS OF MONOSUBSTITUTED CYCLOHEXANES The most stable conformation is a chair with the. The Cyclohexane Chair Flip Master Organic Chemistry.
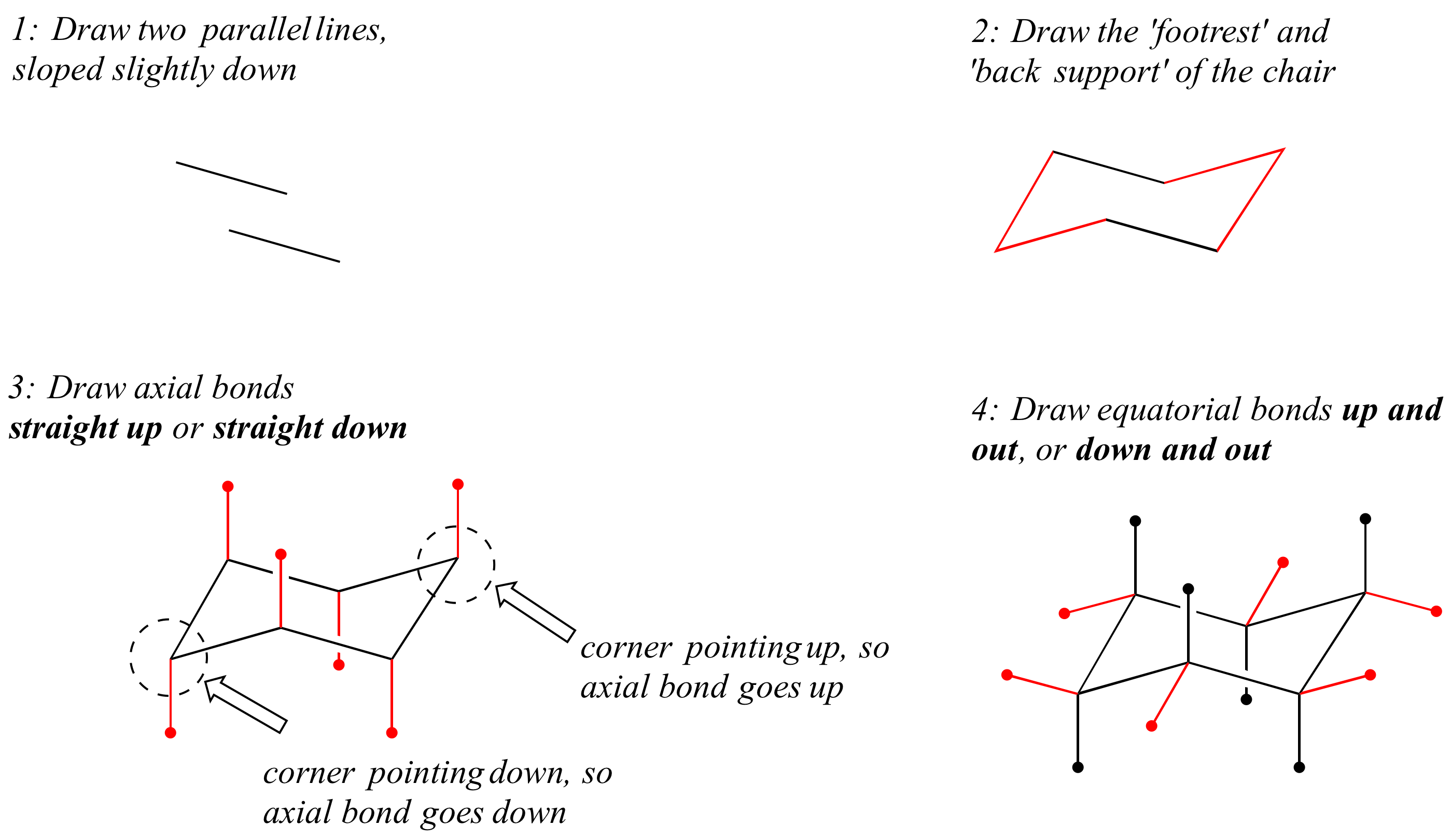
The bridging carbon at the apex of the chair is sketched in step 2. The bridging carbon in the back is drawn in step 3. In the ring-flipping process the chair goes through the following conformations. A potential energy diagram for nng inversion m cyclohexane is shown m Figure 3 18 In the first step the chair conformation is converted to a skew boat which then proceeds to the inverted chair m the second step The skew boat conformation is an inter mediate in the process of ring inversion Unlike a transition state an intermediate is not a. Substituents represented on wedges are always positioned. However due to the cyclohexane chair flip only one signal is seen for a solution of cyclohexane at room temperature as the axial and equatorial proton rapidly interconvert relative to the NMR time scale. 3 6 Conformations Of Cyclic Alkanes Chemistry Libretexts.
The overall process of converting one chair conformation to another chair conformation is known as RING INVERSION or ring flipping and is a very rapid process. It can convert to a twist boat comformation and then to a new chair conformation in a process termed ring-flipping as shown Figure 614 not all the hydrogens are shown for clarity. Half-chair boat half-chair to get to the other chair conformation. Cis- is flexible as the axial and equatorial can interconvert chair flapping process Trans- not interconvertable. The chair conformation of cyclohexane is not rigid. The process of ring flipping takes cyclohexane through a conformation called the twist chair or the half chair form and this form is 108kcal less stable than the chair conformer. What Is The Order Of Stability Of The Conformers Of Cyclohexane Why Quora.

The chair conformation is the most stable conformation of cyclohexane. In step 1 two circles are drawn side-by-side. The cyclohexane ring inverts approximately 10 time a second at room temperature. I n chair cyclohexane there are two types of positions axial and equatorial. 3 kcalmol lower than cis. So the stability increases from Half chair to boat to twist boat and finally the chair conformation. Configuration And Preferred Half Chair Conformation Of The Two Download Scientific Diagram.

CONFORMATIONAL ANALYSIS OF MONOSUBSTITUTED CYCLOHEXANES The most stable conformation is a chair with the. Hydrogen atoms in the chair conformation fall into two sets. In the ring-flipping process the chair goes through the following conformations. Draw the second chair conformation ring-flip-check this post if not sure. Draw this other chair conformation of methylcyclohexane. Disk-like structure- rigid. Calculate Dh For The Process Of Going From Clutch Prep.
The bridging carbon at the apex of the chair is sketched in step 2. The chair conformation is the most stable conformation of cyclohexane. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause steric strain. Disk-like structure- rigid. Generalize as to what chair flipping does to substituents in terms of their equatorialaxial status. The chair conformation of cyclohexane is not rigid. 4 6 Axial And Equatorial Bonds In Cyclohexane Chemistry Libretexts.

Hydrogen atoms in the chair conformation fall into two sets. However due to the cyclohexane chair flip only one signal is seen for a solution of cyclohexane at room temperature as the axial and equatorial proton rapidly interconvert relative to the NMR time scale. Step 4 illustrates the addition of. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. CONFORMATIONAL ANALYSIS OF MONOSUBSTITUTED CYCLOHEXANES The most stable conformation is a chair with the. Number the ring and draw any chair conformation of the compound. Cena In Its Two Stable Conformations A The 2 H 3 Half Chair Download Scientific Diagram.

An alternate conformation for a six-membered ring is called the boat. In this process the equa-torial hydrogens have become axial and the axial hydrogens have become equatorial. Generalize as to what chair flipping does to substituents in terms of their equatorialaxial status. In the ring-flipping process C. Of the rightmost carbon changes one chair conformation into another completely equivalent chair conformation. Then take C-4 and invert it to remake a chair conformation. Ring Flip Wikiwand.

Number the ring and draw any chair conformation of the compound. In step 1 two circles are drawn side-by-side. 3 kcalmol lower than cis. This process inverts the chair conformation and is called chair flipping See figure below. Two 1 H NMR signals should be observed in principle corresponding to axial and equatorial protons. Cis- is flexible as the axial and equatorial can interconvert chair flapping process Trans- not interconvertable. How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube.

Disk-like structure- rigid. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. The chair conformation of cyclohexane is not rigid. In ad-dition up carbons have become down carbons and vice versa. Scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. When viewed along any carboncarbon bond viewing the structure from an end Fig2 the bonds are seen to be perfectly staggered. Ring Flipping An Overview Sciencedirect Topics.

However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. Moreover the hydrogen atoms at opposite corners. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. Upon ring flipping the axial and equatorial bonds interchange their positions. Hydrogen atoms in the chair conformation fall into two sets. Chemical Structures Of Benzene Top And Cyclohexane Bottom Benzene Download Scientific Diagram.

This process inverts the chair conformation and is called chair flipping See figure below. Two 1 H NMR signals should be observed in principle corresponding to axial and equatorial protons. Generalize as to what chair flipping does to substituents in terms of their equatorialaxial status. The bridging carbon at the apex of the chair is sketched in step 2. In ad-dition up carbons have become down carbons and vice versa. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. Cyclohexane Conformations A Top Chair Conformation B Middle Download Scientific Diagram.
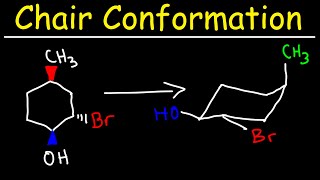
Then take C-4 and invert it to remake a chair conformation. The chair conformation of cyclohexane is not rigid. Show this interconversion by drawing the two chair conformations and putting a reversible arrow. In this process the equa-torial hydrogens have become axial and the axial hydrogens have become equatorial. This process inverts the chair conformation and is called chair flipping See figure below. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. Chair Conformation And Ring Flips Youtube.











