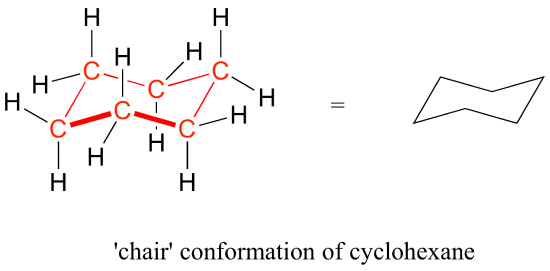
chair conformation definition Bruice Organic Chemistry 6th Edition Chapters 21-215 33-35 51-58 511-513 517 520-521. The chair conformation is the most stable conformer.
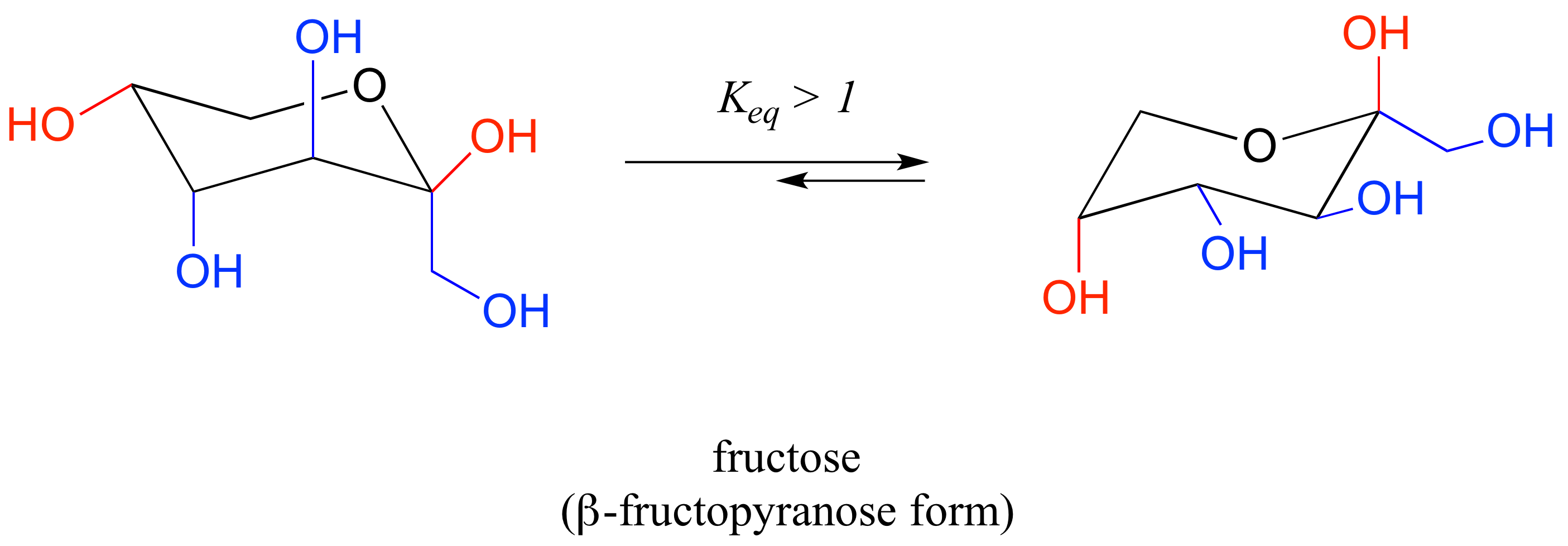
Chair Conformation Definition, The final piece of these conversions is often to draw a complete chair conformation of the pyranose. 10 conformations of cyclohexane all of which are free from angle strain. Noun the most stable chemical conformation of a six-membered single bonded carbon ring such cyclohexane Show declension of chair conformation chair conformation plural chair conformations.
 Chair Conformations Examples Youtube From youtube.com
Chair Conformations Examples Youtube From youtube.com
Converting Haworth to Chair. Number the ring and draw any chair conformation of the compound. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. 10 conformations of cyclohexane all of which are free from angle strain.
The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered.
The 13 describes the distance between the substituent and hydrogens that are in axial position. The different conformations are called conformers a blend of the words conformation and isomer. Half-chair conformation - The high-energy intermediate conformation of cyclohexane as it converts from one chair conformation into the other. Overview of Chair Conformation Of Glucose A simple form of sugar that has a general formula of C 6 H 12 O 6 C_6H_12O_6 C 6 H 1 2 O 6 is called glucose. Chair conformations are commonly used to describe the various interactions between atoms on cycylohexanes.
Another Article :
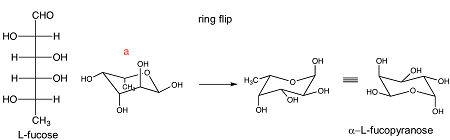
The final piece of these conversions is often to draw a complete chair conformation of the pyranose. If playback doesnt begin shortly try restarting your device. The chair conformation is the most stable conformer. What does chair-conformation mean. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. In the first conformer we have two chlorines in axial positions so the total steric strain is. Draw Chair Conformations From The Fischer Projection In Step A Why Is The Methyl Group Pointing Downwards And Why Do We Need To Do Ring Flips For This Particular Sugar To Get.

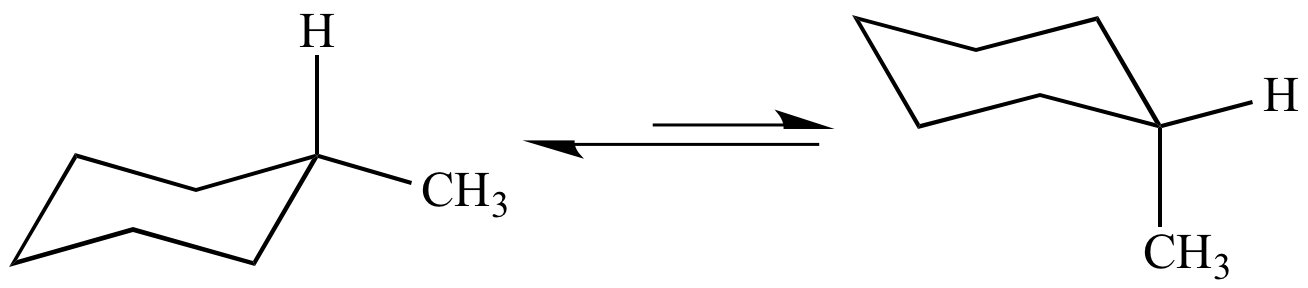
- footrest flips upward forms the boat. Draw the second chair conformation ring-flip-check this post if not sure. Noun the most stable chemical conformation of a six-membered single bonded carbon ring such cyclohexane Show declension of chair conformation chair conformation plural chair conformations. In the first conformer we have two chlorines in axial positions so the total steric strain is. Conformational isomers - those that rapidly interconvert at. Number the ring and draw any chair conformation of the compound. Organic Chemistry Stereoisomerism Of Chair Conformation Youtube.
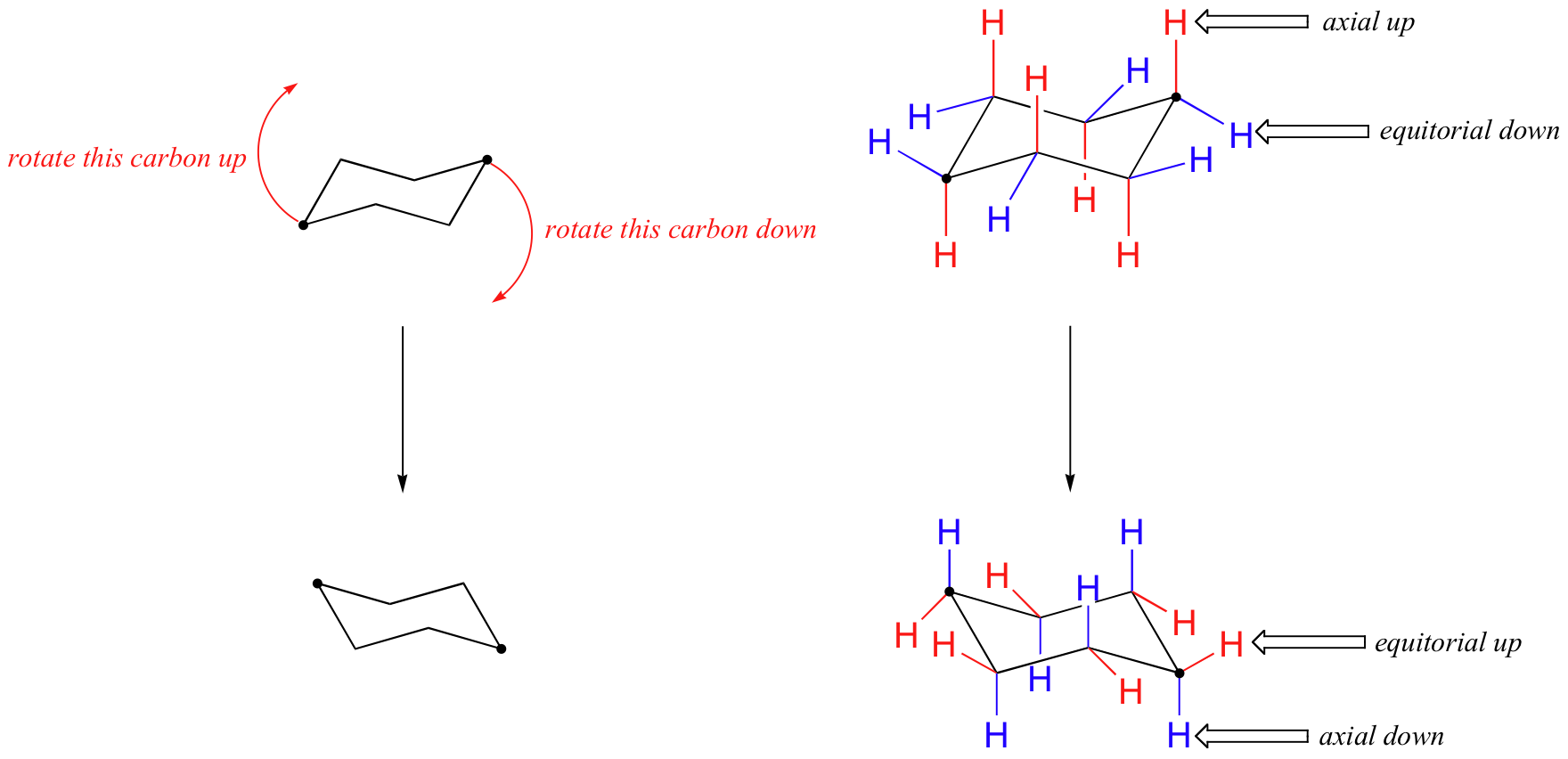
Chair conformation plural chair conformations the most stable chemical conformation of a six-membered single bonded carbon ring such as cyclohexane. Number the ring and draw any chair conformation of the compound. Chair Conformations Examples - YouTube. A The two chair conformations are of equal energy. In the first conformer we have two chlorines in axial positions so the total steric strain is. Stereochemistry - the arrangement of atoms in space. 4 6 Axial And Equatiorial Bonds In Cyclohexane Chemistry Libretexts.
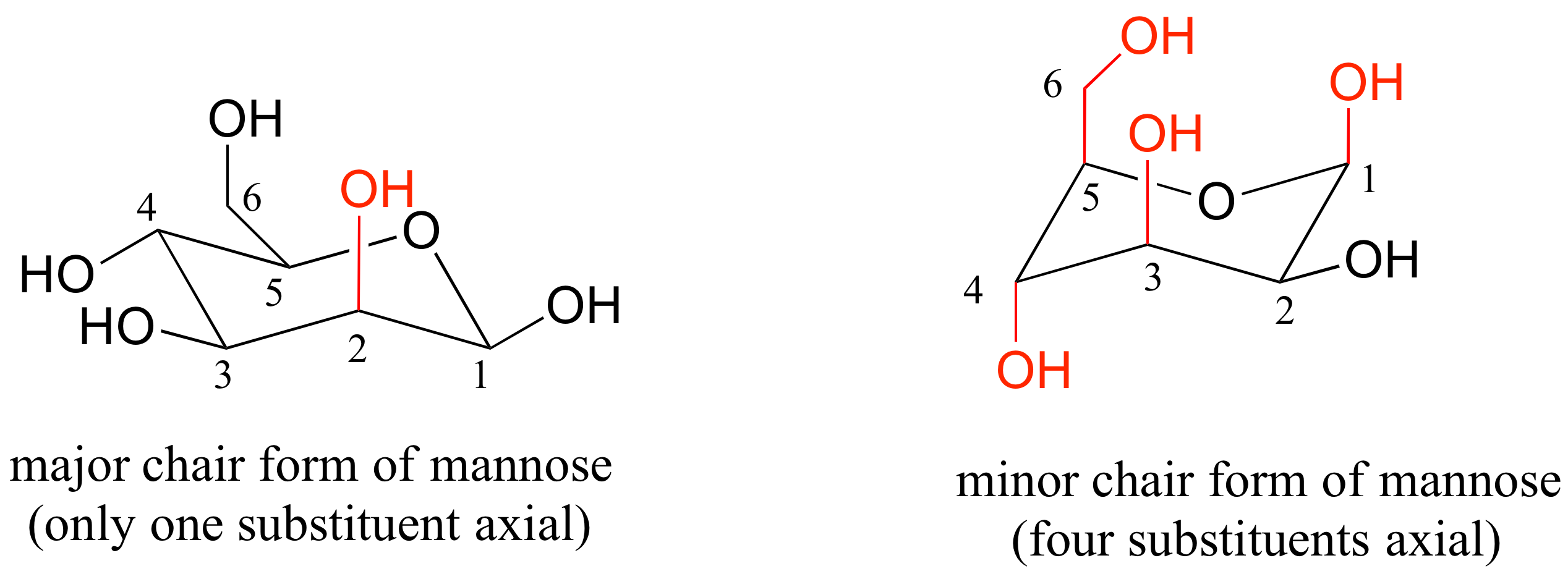
The chair conformation is the most stable conformation of cyclohexane Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring The bond angles in this conformation are 1109. Stereochemistry - the arrangement of atoms in space. It involves rotating one of the dihedrals to zero such that four adjacent atoms are coplanar and the other two atoms are out of plane one above and one below. The most stable conformation of a glucose molecule is called the chair conformation of glucose. In cyclohexane derivatives the two chair conformers interconvert with rapidly at room temperature with cyclohexane itself undergoing the ring-flip at a rates of approximately 10 5 ring-flipssec with an overall energy barrier of 10 kcalmol 42 kJmol which precludes their separation at ambient temperatures. Number the ring and draw any chair conformation of the compound. 3 13 Solutions To Chapter 3 Exercises Chemistry Libretexts.

If playback doesnt begin shortly try restarting your device. The final piece of these conversions is often to draw a complete chair conformation of the pyranose. And now the stabilities. Half-chair conformation - The high-energy intermediate conformation of cyclohexane as it converts from one chair conformation into the other. Definition of chair boat twist. The chair conformation is the most stable conformation of cyclohexane. Draw The Chair Conformations Of Each Of The Following Molecules Indicating Which One Should Be More Stable And Briefly Justify Your Reasoning Study Com.
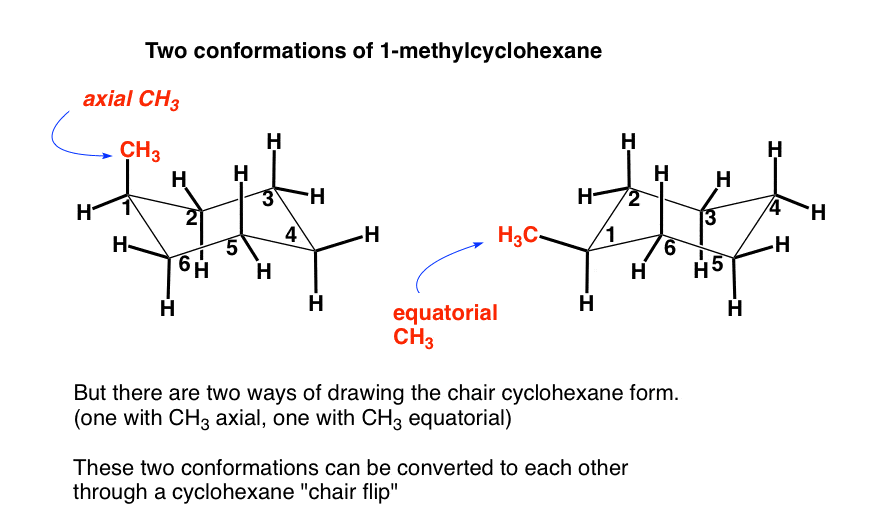
Definition of chair boat twist. Boat conformation ring flip axial equatorial equial. Chair conformations are commonly used to describe the various interactions between atoms on cycylohexanes. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. Half-chair conformation - The high-energy intermediate conformation of cyclohexane as it converts from one chair conformation into the other. Definition of chair boat twist. The Cyclohexane Chair Flip Master Organic Chemistry.
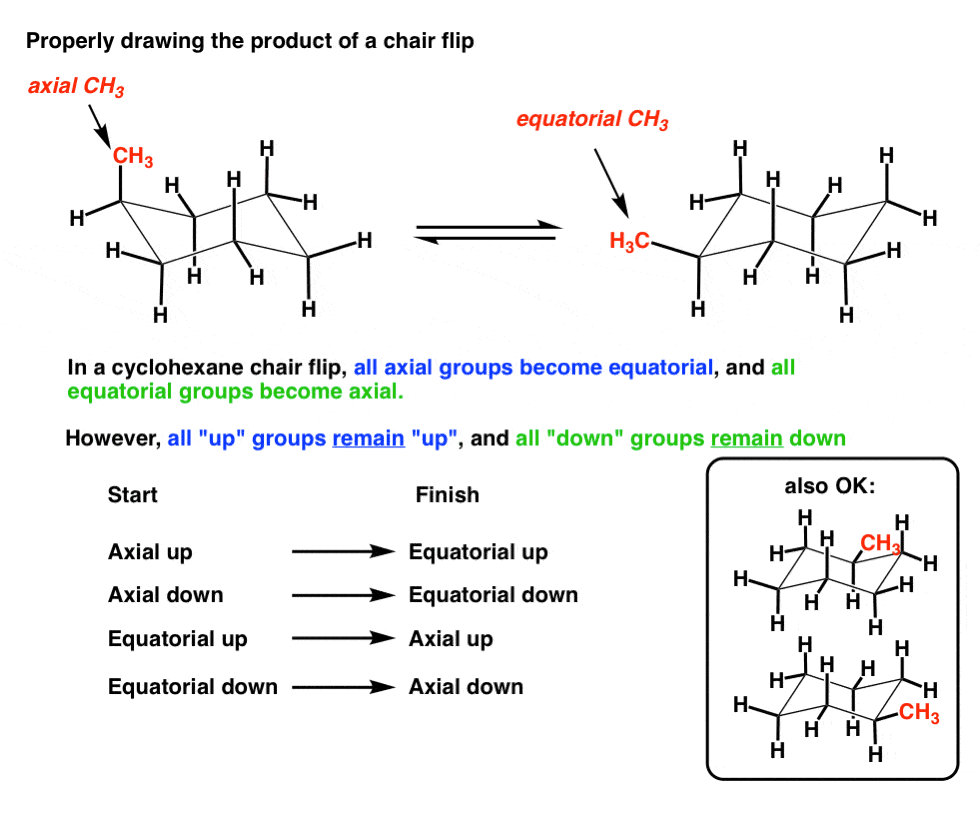
Explaining how A-Values are related to cyclohexane flip energy. Converting Haworth to Chair. The most stable conformation of a glucose molecule is called the chair conformation of glucose. Anti left and syn center. Chair conformations are commonly used to describe the various interactions between atoms on cycylohexanes. Chair conformation is the most stable with the lowest energy and there is no steric or torsional tension. The Cyclohexane Chair Flip Master Organic Chemistry.
Explaining how A-Values are related to cyclohexane flip energy. D The higher energy chair conformation contains two axial methyl groups. The final piece of these conversions is often to draw a complete chair conformation of the pyranose. For each chair conformer add the energy of all the groups on axial position. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. 1 2 Conformations Of Cyclic Organic Molecules Chemistry Libretexts.

The different conformations are called conformers a blend of the words conformation and isomer. In the first conformer we have two chlorines in axial positions so the total steric strain is. Chair conformation is the most stable with the lowest energy and there is no steric or torsional tension. Definition of chair boat twist. For each chair conformer add the energy of all the groups on axial position. D The higher energy chair conformation contains two axial methyl groups. Chair Conformations Examples Youtube.

The trick is to remember that just like the Haworth projections the chair conformations also have the well-defined up and down positions. Conformationalisomers or conformersor rotational isomersor rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. Overview of Chair Conformation Of Glucose A simple form of sugar that has a general formula of C 6 H 12 O 6 C_6H_12O_6 C 6 H 1 2 O 6 is called glucose. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. Bruice Organic Chemistry 6th Edition Chapters 21-215 33-35 51-58 511-513 517 520-521. Conformational isomers - those that rapidly interconvert at. Chair Conformation And Ring Flips Youtube.
In the first conformer we have two chlorines in axial positions so the total steric strain is. Number the ring and draw any chair conformation of the compound. Explaining how A-Values are related to cyclohexane flip energy. If carbon atoms 1 2 4 and 5 of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the plane the conformation of symmetry group D3d is called a chair form. Such a definition are minimised by the fact that in general only a few of the possible conformations are energetically preferred. Calculating Flip Energy. 3 2 Conformations Of Cyclic Organic Molecules Chemistry Libretexts.
In the first conformer we have two chlorines in axial positions so the total steric strain is. The 13 describes the distance between the substituent and hydrogens that are in axial position. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. A The two chair conformations are of equal energy. Stereochemistry - the arrangement of atoms in space. Number the ring and draw any chair conformation of the compound. Illustrated Glossary Of Organic Chemistry Chair Conformation.

At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. For each chair conformer add the energy of all the groups on axial position. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. B The higher energy chair conformation contains one axial methyl group and one equatorial methyl group. Half-chair conformation - The high-energy intermediate conformation of cyclohexane as it converts from one chair conformation into the other. Chair conformation is the most stable with the lowest energy and there is no steric or torsional tension. Difference Between Chair And Boat Conformation Compare The Difference Between Similar Terms.

One may therefore con-sider chair boat and twist-boat a conformation half-way between two boats. In the first conformer we have two chlorines in axial positions so the total steric strain is. Such a definition are minimised by the fact that in general only a few of the possible conformations are energetically preferred. Chair conformation is the most stable with the lowest energy and there is no steric or torsional tension. The chair conformation is the most stable conformation of cyclohexane Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring The bond angles in this conformation are 1109. Noun the most stable chemical conformation of a six-membered single bonded carbon ring such cyclohexane Show declension of chair conformation chair conformation plural chair conformations. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.

Explaining how A-Values are related to cyclohexane flip energy. Chair conformations are commonly used to describe the various interactions between atoms on cycylohexanes. Conformationalisomers or conformersor rotational isomersor rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. B The higher energy chair conformation contains one axial methyl group and one equatorial methyl group. Explaining how A-Values are related to cyclohexane flip energy. How To Draw Chair Conformations.










