chair conformation rotation We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. This proceeds from one chair to twist boat to boat to twist boat to the other chair conformation.
Chair Conformation Rotation, Look through each of the C-C bonds in the cyclohexane ring. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest.
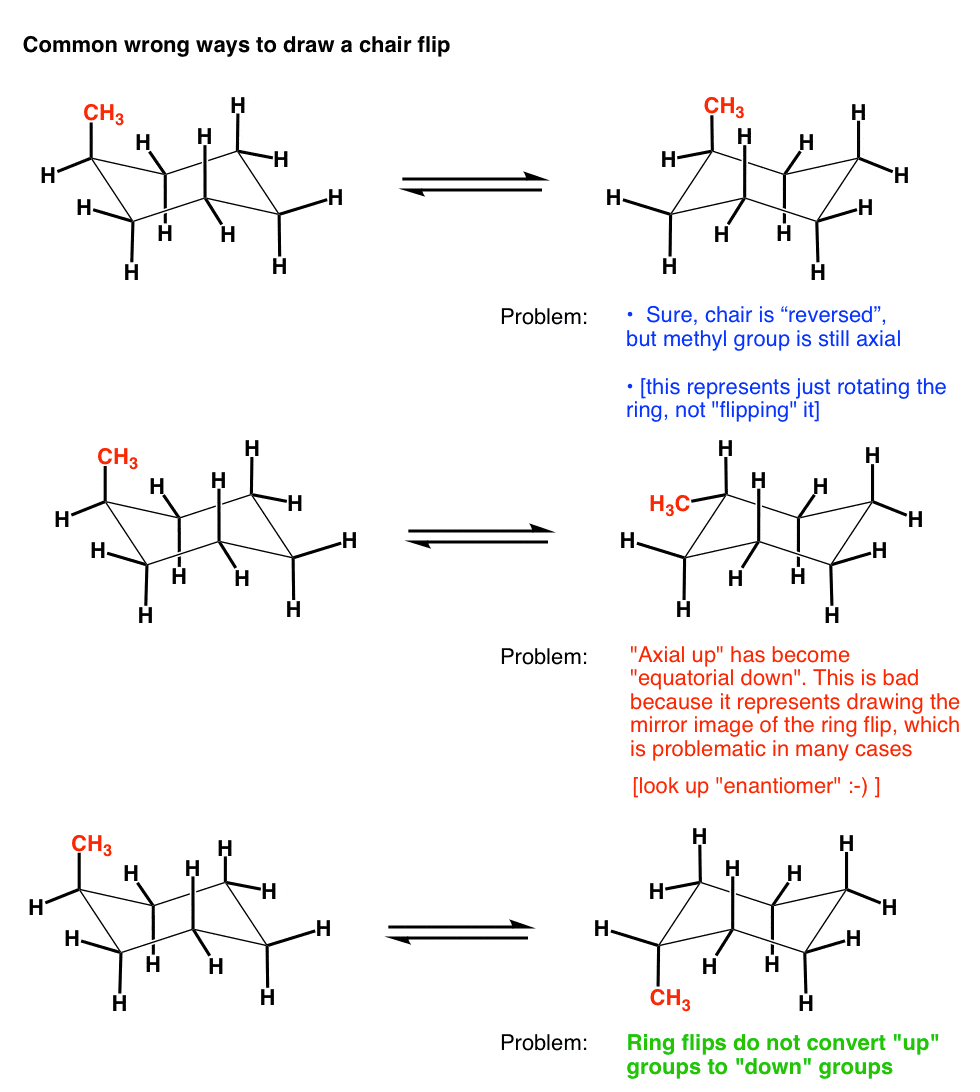
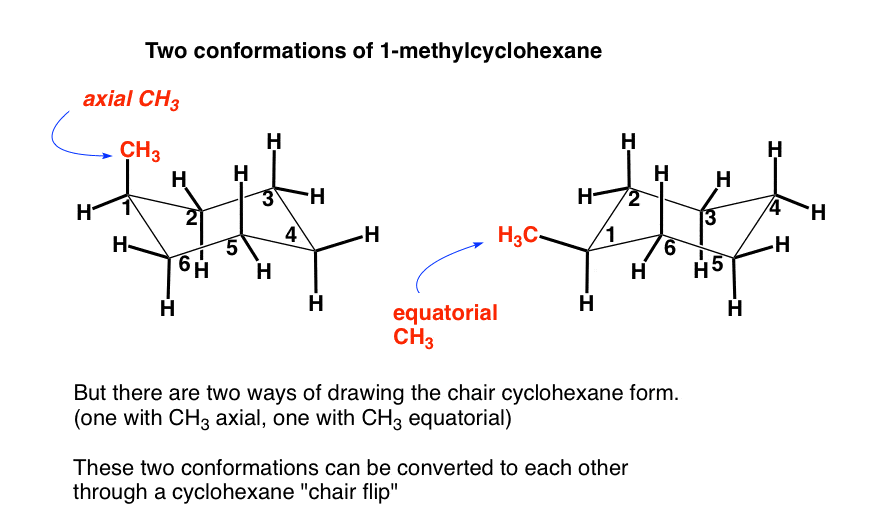 The Cyclohexane Chair Flip Master Organic Chemistry From masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry From masterorganicchemistry.com
The chair conformation cannot deform without changing the bond angles or lengths. The chair conformation is perfectly staggered about all C-C-C bonds and therefore there is no torsional strain. The chair and boat conformations of cyclohexane are virtually angle strain free. Conformational rotation of cyclohexane interconverts the conformations. This is true for 1R-33-dichlorocyclohexanol.
Look through each of the C-C bonds in the cyclohexane ring.
Cyclohexane rapidly interconverts between two stable chair conformations because of the ease of. 12 From the chair conformation rotate about the carbon-carbon bonds of the ring to form a boat conformation. All the carbon-hydrogen bonds are also fully staggered eliminating the torsional strain. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. Chair Boat Chair Click the structures and reaction arrows to view the 3D models and animations respectively Cyclic alkanes can also interconvert between their conformers by rotation of the single carbon to carbon bonds.
Another Article :

Be sure to verify this with models. Conformational rotation of cyclohexane interconverts the conformations. The chair conformation is a six-membered ring in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. So the 13-diaxial notation is the most common way we refer to the gauche interactions of axial groups in the chair conformations. In the boat conformation carbon atoms 1 and 4 are both above they could also both be below the plane described by carbon atoms 2 3 5 and 6. Cyclohexane rapidly interconverts between two stable chair conformations because of the ease of. The Meso Trap Master Organic Chemistry Organic Chemistry Organic Chemistry Books Organic Chemistry Study.

About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. This is true for 1R-33-dichlorocyclohexanol. The chair conformation cannot deform without changing the bond angles or lengths. Conformational rotation of cyclohexane interconverts the conformations. Position your model so that the CH3 group is equatorial. In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. Pin On Alkene Reactions With Practice Problems.
The boat conformation has both steric strain due to interaction of flagpole hydrogens and torsional strain. This proceeds from one chair to twist boat to boat to twist boat to the other chair conformation. Chair Conformation Interconversions A ring flip is a phenomenon involving the interconversion by rotation around the C-C bonds of cyclic conformers. The boat conformation has both steric strain due to interaction of flagpole hydrogens and torsional strain. When a cyclohexane ring undergoes a chair-chair conformation conversion that is known as ring flippingRing flipping comes from C-C bond rotation but since all of the bonds are limited within the ring the rotation can only occur partially which leads to the ring flipping. Together these features make the chair conformation. 3 13 Solutions To Chapter 3 Exercises Chemistry Libretexts.
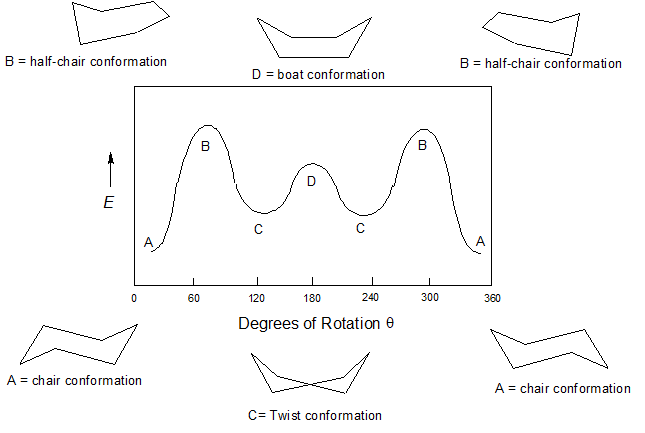
Together these features make the chair conformation very stable. Look through each of the C-C bonds in the cyclohexane ring. The most stable conformation is the β chair conformation since it reduces steric hindrance or electron repulsion among the bulky groups. If your first chair has the upper line on the right draw the second chair with the upper line left. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. This proceeds from one chair to twist boat to boat to twist boat to the other chair conformation. 4 7 Cyclohexane Conformations Chemistry Libretexts.

Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. Add the CH3 and hydrogen atoms to the drawing for this conformer. In the boat conformation carbon atoms 1 and 4 are both above they could also both be below the plane described by carbon atoms 2 3 5 and 6. The chair conformation cannot deform without changing the bond angles or lengths. If your first chair has the upper line on the right draw the second chair with the upper line left. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. Pin On Alkene Reactions With Practice Problems.

Together these features make the chair conformation very stable. In the boat conformation carbon atoms 1 and 4 are both above they could also both be below the plane described by carbon atoms 2 3 5 and 6. 21 In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. Once you have your first chair determine if your parallel lines have upper right or upper left. Generally the axial conformation of a given cyclohexane is less stable than the corresponding equatorial conformation. Chair Conformation Interconversions A ring flip is a phenomenon involving the interconversion by rotation around the C-C bonds of cyclic conformers. Chair Conformation Chirality Chemistry Stack Exchange.
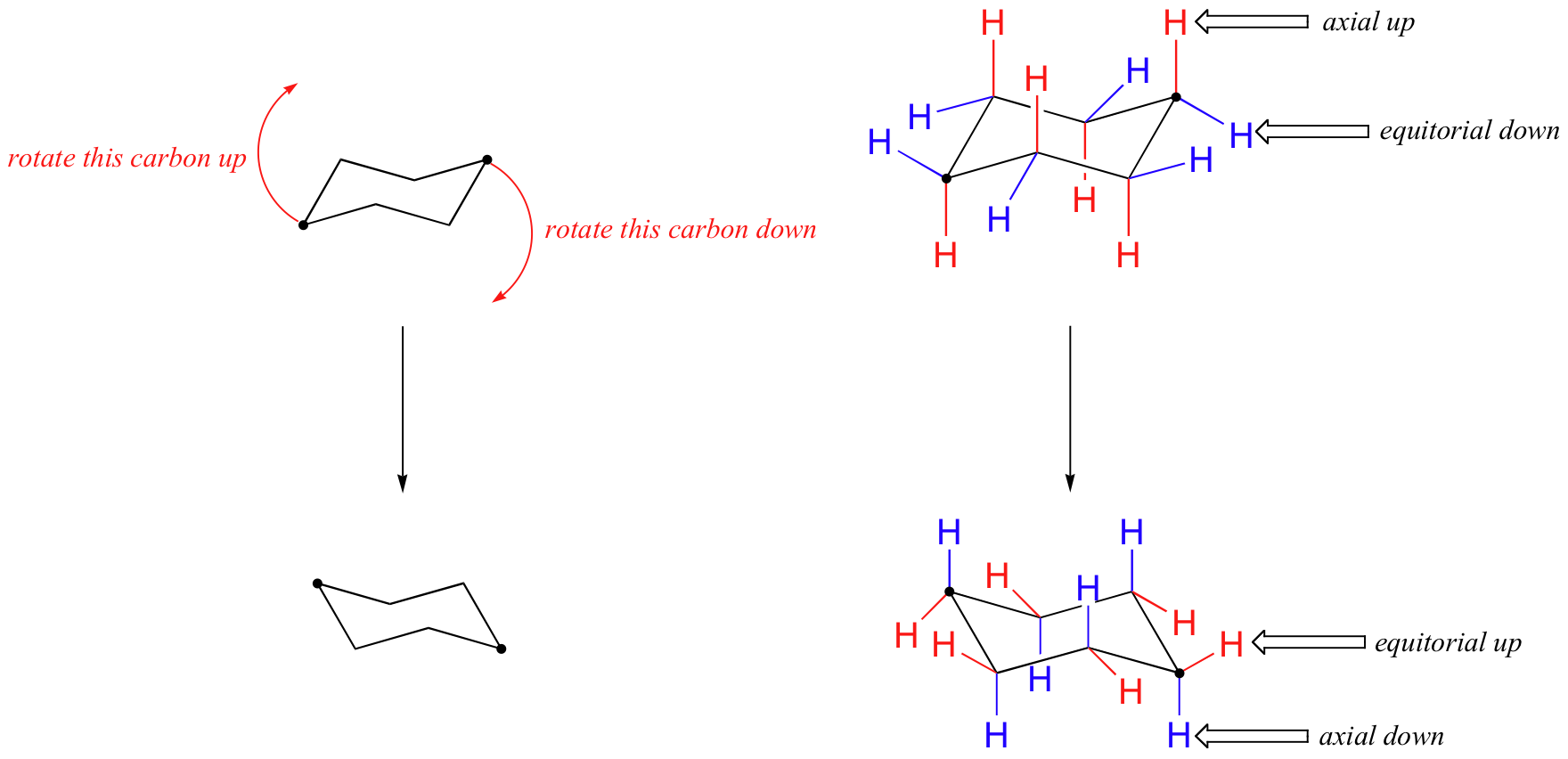
The chair conformation cannot deform without changing the bond angles or lengths. Chair Conformation Interconversions A ring flip is a phenomenon involving the interconversion by rotation around the C-C bonds of cyclic conformers. If your first chair has the upper line on the right draw the second chair with the upper line left. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The most stable conformation is the β chair conformation since it reduces steric hindrance or electron repulsion among the bulky groups. In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. 4 7 Cyclohexane Conformations Chemistry Libretexts.

Chair Boat Chair Click the structures and reaction arrows to view the 3D models and animations respectively Cyclic alkanes can also interconvert between their conformers by rotation of the single carbon to carbon bonds. Chair Boat Chair Click the structures and reaction arrows to view the 3D models and animations respectively Cyclic alkanes can also interconvert between their conformers by rotation of the single carbon to carbon bonds. When a cyclohexane ring undergoes a chair-chair conformation conversion that is known as ring flippingRing flipping comes from C-C bond rotation but since all of the bonds are limited within the ring the rotation can only occur partially which leads to the ring flipping. Generally the axial conformation of a given cyclohexane is less stable than the corresponding equatorial conformation. A rotation of either perspective by an odd multiple of 60 that is 60 180 and so on followed by the slight shift in viewing direction gives the other perspective. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Isomersim Scheme Enantiomers Constitutional Isomers Diastreomers Organic Chemistry Study Chemistry Biology Notes.
The chair conformation is a six-membered ring in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. The chair conformation is a six-membered ring in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. All the carbon-hydrogen bonds are also fully staggered eliminating the torsional strain. Once you have your first chair determine if your parallel lines have upper right or upper left. In the boat conformation carbon atoms 1 and 4 are both above they could also both be below the plane described by carbon atoms 2 3 5 and 6. The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle. A The Chair Conformation Of Cyclohexane B Representation Of The Download Scientific Diagram.
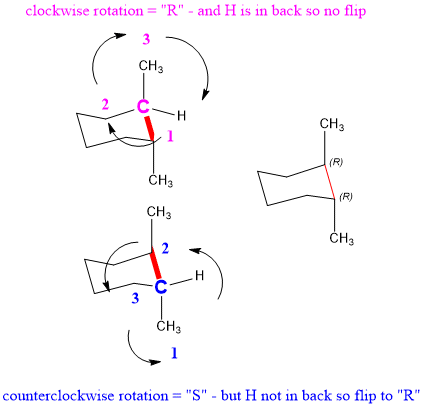
Look through each of the C-C bonds in the cyclohexane ring. When a cyclohexane ring undergoes a chair-chair conformation conversion that is known as ring flippingRing flipping comes from C-C bond rotation but since all of the bonds are limited within the ring the rotation can only occur partially which leads to the ring flipping. Together these features make the chair conformation very stable. It is important for you to be able to draw a cyclohexane chair conformation. Chair Conformation Interconversions A ring flip is a phenomenon involving the interconversion by rotation around the C-C bonds of cyclic conformers. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Rotation Organic Chemistry Help.

12 From the chair conformation rotate about the carbon-carbon bonds of the ring to form a boat conformation. 21 In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees. With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees. The chair and boat conformations of cyclohexane are virtually angle strain free. The chair conformation is a six-membered ring in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. The Cyclohexane Chair Flip Master Organic Chemistry.

Draw another chair using the steps described above but change the direction of your top line. A rotation of either perspective by an odd multiple of 60 that is 60 180 and so on followed by the slight shift in viewing direction gives the other perspective. The chair conformation cannot deform without changing the bond angles or lengths. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. 21 In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. Be sure to verify this with models. Pin On Tukimica.
So the 13-diaxial notation is the most common way we refer to the gauche interactions of axial groups in the chair conformations. The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle. The chair conformation cannot deform without changing the bond angles or lengths. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. 12 From the chair conformation rotate about the carbon-carbon bonds of the ring to form a boat conformation. Add the CH3 and hydrogen atoms to the drawing for this conformer. The Cyclohexane Chair Flip Master Organic Chemistry.

Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top. Look through each of the C-C bonds in the cyclohexane ring. When a cyclohexane ring undergoes a chair-chair conformation conversion that is known as ring flippingRing flipping comes from C-C bond rotation but since all of the bonds are limited within the ring the rotation can only occur partially which leads to the ring flipping. 21 In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. MUTAROTATION Is the special rotation of. Position your model so that the CH3 group is equatorial. Alkane Conformations And Nomenclature.

MUTAROTATION Is the special rotation of. Chair Conformation Interconversions A ring flip is a phenomenon involving the interconversion by rotation around the C-C bonds of cyclic conformers. A rotation of either perspective by an odd multiple of 60 that is 60 180 and so on followed by the slight shift in viewing direction gives the other perspective. Cyclohexane rapidly interconverts between two stable chair conformations because of the ease of. The chair and boat conformations of cyclohexane are virtually angle strain free. 12 From the chair conformation rotate about the carbon-carbon bonds of the ring to form a boat conformation. The Covalent Bond Mcat Review Organic Chemistry Organic Chemistry Study Chemistry Lessons.











