chair conformation plane Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. The twist-boat or skew conformation has point group D 2 and is indeed chiral.
Chair Conformation Plane, Cyclohexane chair D. D 3d contains 3C 2 perpendicular to C 3 with 3σ S 6 axis and a centre of inversion. This will represent the new conformation as it should look if the ring flips.
 Organic Chemistry Chair Conformations Youtube From youtube.com
Organic Chemistry Chair Conformations Youtube From youtube.com
In the chair conformation the reference plane is chosen such that the lowest-numbered atom usually C-1 is exoplanar. There may be the presence of substituents for. This is your first axial substituent. In the skew conformation the plane contains three adjacent atoms and one other with the atom with the lowest possible number exoplanar. With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees.
In this con-formation of cyclohexane the carbons do not lie in a single plane.
A six-membered ring conformation in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. However only one conformation is the lowest energy highest stability chair. This is the predominant structure adopted by molecules of cyclohexane. The twist-boat or skew conformation has point group D 2 and is indeed chiral. Cyclohexane chair D 3d.
Another Article :

8 to be exact. With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees. Rather the carbon skele-ton is puckered. Almost all of your work with cyclohexanes will involve chair conformations. Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. The chair conformation is a six-membered ring in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube.
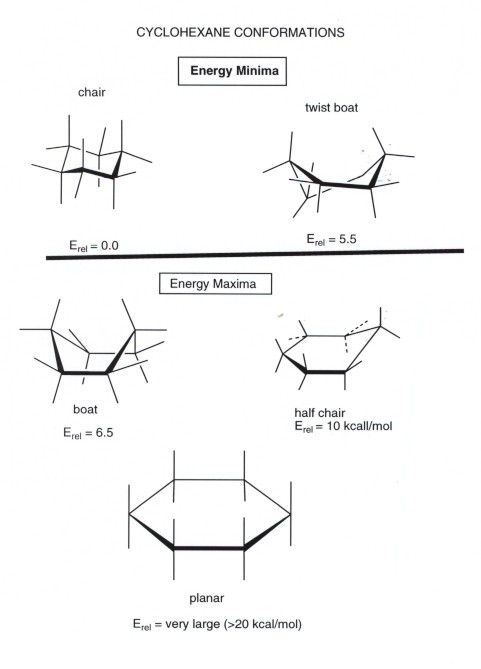
These two chair conformations are the most common and comfortable of all the conformational possibilities available to a cyclohexane ring. Cyclohexane chair D. Drawing the Chair Flipped Conformation. The methyl groups all go in equatorial positions. Cyclohexane in the chair conformation has a C3 axis perpendicular to the average plane of the ring three perpendicular C 2 axes between the carbons and three v planes each including the C 3. Almost all of your work with cyclohexanes will involve chair conformations. Cyclohexane Conformational Analysis.

With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees. These two chair conformations are the most common and comfortable of all the conformational possibilities available to a cyclohexane ring. Chair conformation is the term used in organic chemistry that represents the chair-like structure of a carbon ring consisting of six carbon atoms. Put a rectangle and name. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. Carbon 1 which was an up carbon is now a down carbon. Axial And Equatiorial Bonds In Cyclohexane Mcc Organic Chemistry.
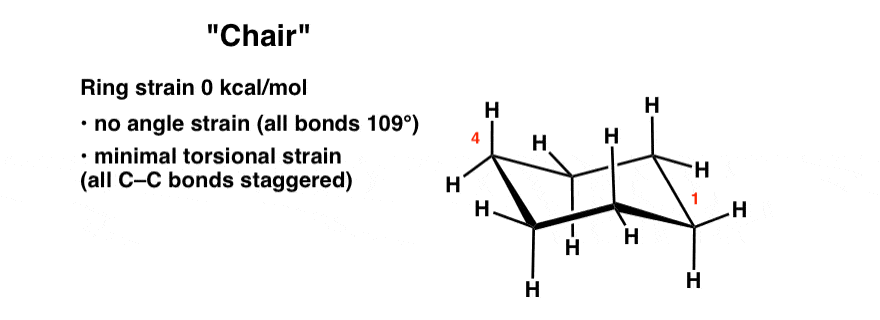
Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. 1R 2R 4R 124-trimethylcyclohexane does not have the methyl groups in the same plane. The most stable conformation of cyclohexane is shown in Fig. A six-membered ring conformation in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. All the carbon-hydrogen bonds are also fully staggered eliminating the torsional strain. Chair conformation is the term used in organic chemistry that represents the chair-like structure of a carbon ring consisting of six carbon atoms. Cyclohexane Conformations Master Organic Chemistry.
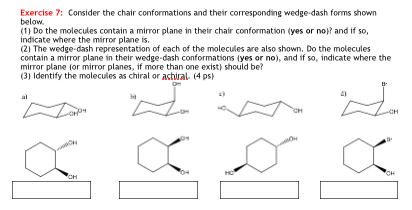
In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. The twist-boat or skew conformation has point group D 2 and is indeed chiral. However only one conformation is the lowest energy highest stability chair. 8 to be exact. Atoms above the plane are written before the conformer label as a superscript. With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees. Solved Exercise 7 Consider The Chair Conformations And Chegg Com.

Carbon 4 which was down is now. Rather the carbon skele-ton is puckered. This means that there are a number of combinations. Finally by lifting one carbon above the ring plane and the other below the plane a relatively strain-free chair conformer is formed. These two chair conformations are the most common and comfortable of all the conformational possibilities available to a cyclohexane ring. Cyclohexane chair D. Stereoisomers.
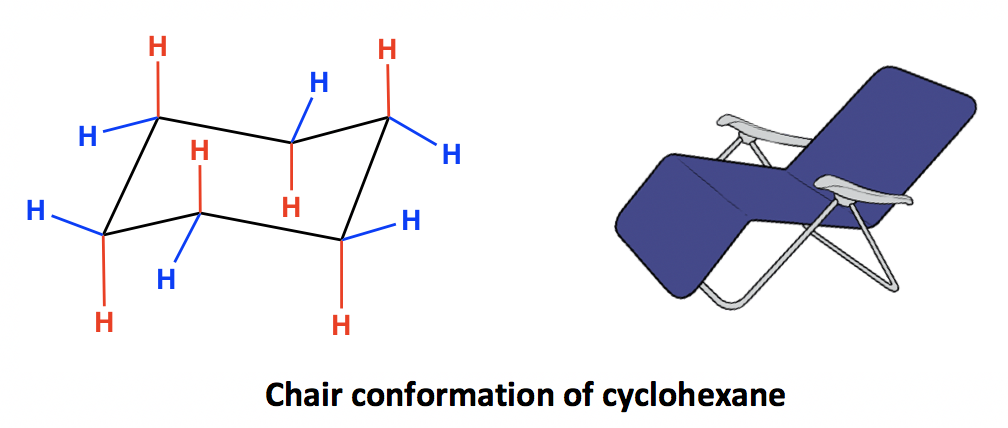
To draw the chair flip conformation you have to redraw the ring as its mirror image. D 3d contains 3C 2 perpendicular to C 3 with 3σ S 6 axis and a centre of inversion. 8 to be exact. 1 model in this collection. C-H axis is parallel to the plane of the ring. The chair conformation cannot deform without changing the bond angles or lengths. 4 3 Conformation Analysis Of Cyclohexane Organic Chemistry.
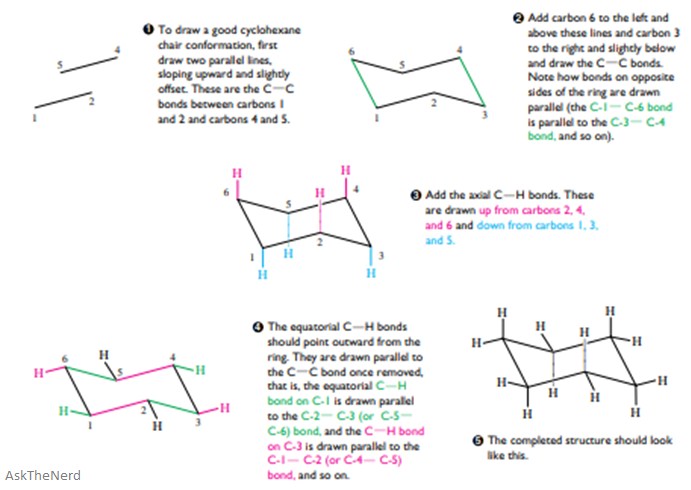
Axial and Equatorial Bonds in Cyclohexane Chair cyclohexane has two types of hydrogens. Carbon 4 which was down is now. Chair conformation is the term used in organic chemistry that represents the chair-like structure of a carbon ring consisting of six carbon atoms. This will represent the new conformation as it should look if the ring flips. The boat and chair conformations are indeed symmetric and achiral. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. Axial And Equatorial Facts Summary Definition Chemistry Revision.
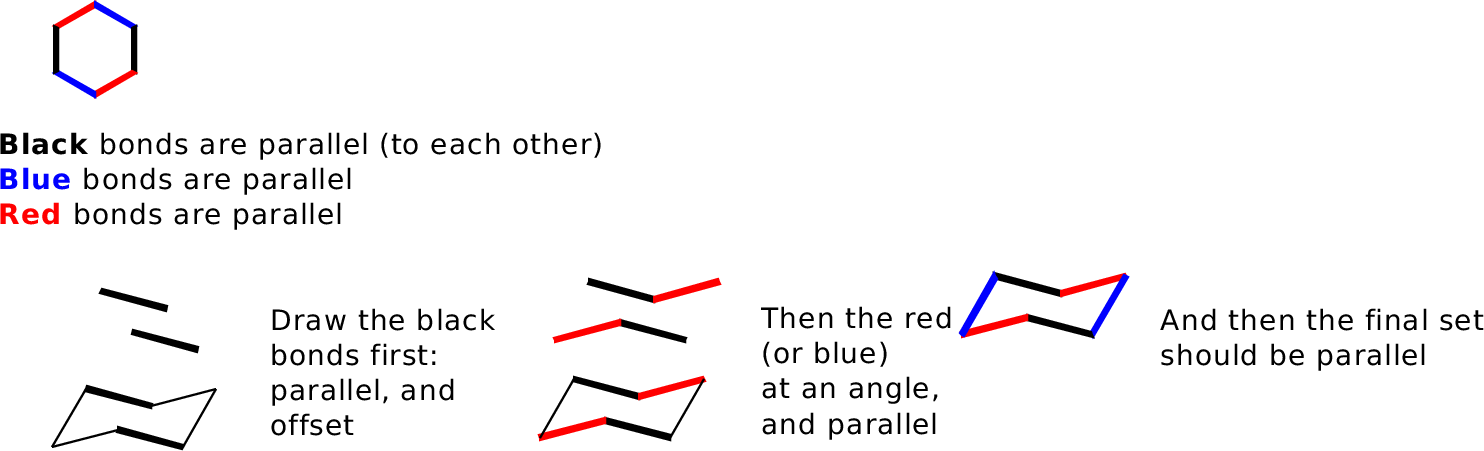
This will represent the new conformation as it should look if the ring flips. 1R 2R 4R 124-trimethylcyclohexane does not have the methyl groups in the same plane. C-H axis is perpendicular to the plane of the ring equatorial. Drawing the Chair Flipped Conformation. A six-membered ring conformation in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. In the chair conformation the reference plane is chosen such that the lowest-numbered atom usually C-1 is exoplanar. Drawing Cyclohexane.
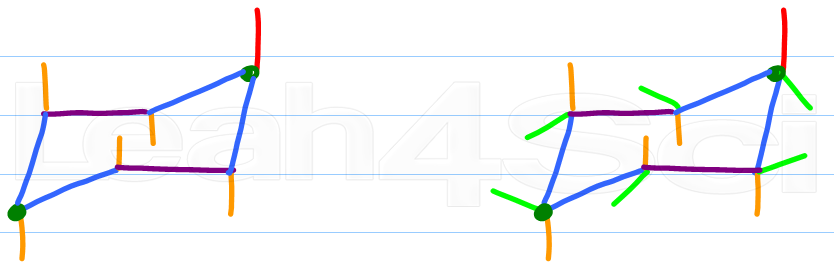
A normal half-chair will have an axis of symmetry and is chiral. C-H axis is perpendicular to the plane of the ring equatorial. This will represent the new conformation as it should look if the ring flips. Almost all of your work with cyclohexanes will involve chair conformations. These two chair conformations are the most common and comfortable of all the conformational possibilities available to a cyclohexane ring. Rather the carbon skele-ton is puckered. Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry.
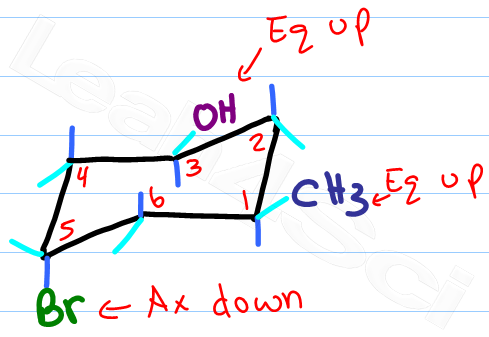
Carbon 1 which was an up carbon is now a down carbon. However only one conformation is the lowest energy highest stability chair. C-H axis is perpendicular to the plane of the ring equatorial. Carbon 1 which was an up carbon is now a down carbon. 1 model in this collection. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion. Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry.

This means that there are a number of combinations. C-H axis is perpendicular to the plane of the ring equatorial. Carbon 4 which was down is now. Boat and Twist Boat Conformations There are additional conformations of cyclohexane rings. All the carbon-hydrogen bonds are also fully staggered eliminating the torsional strain. There may be the presence of substituents for. 4 6 Axial And Equatorial Bonds In Cyclohexane Chemistry Libretexts.

We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. If you have not already done so you should. Axial and Equatorial Bonds in Cyclohexane Chair cyclohexane has two types of hydrogens. A normal half-chair will have an axis of symmetry and is chiral. The chair conformation cannot deform without changing the bond angles or lengths. This is your first axial substituent. Cyclohexane Mcc Organic Chemistry.
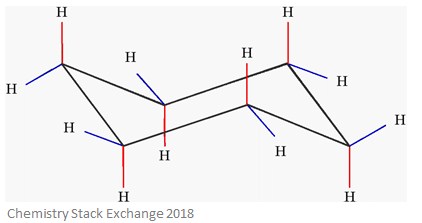
With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees. Finally by lifting one carbon above the ring plane and the other below the plane a relatively strain-free chair conformer is formed. 1 model in this collection. Rather the carbon skele-ton is puckered. Axial and Equatorial Bonds in Cyclohexane Chair cyclohexane has two types of hydrogens. Almost all of your work with cyclohexanes will involve chair conformations. Axial And Equatorial Facts Summary Definition Chemistry Revision.

1 model in this collection. C-H axis is perpendicular to the plane of the ring equatorial. A normal half-chair will have an axis of symmetry and is chiral. All the carbon-hydrogen bonds are also fully staggered eliminating the torsional strain. The A-value of a tert-Butyl is in the range of 5 kcalmol which is around the value of the energy difference between cyclohexane in twist-boat and chair conformation. The boat and chair conformations are indeed symmetric and achiral. Conformational Analysis Of Cyclohexane Stereochemistry Organic Chemistry Youtube.