chair conformation for glucose If playback doesnt begin shortly try. D-Glucose can also be represented in chair form to decide the stability of alpha and beta anomersBeta anomer is more stable because all bulky groups are at.
Chair Conformation For Glucose, Below you can see how to determine what goes up and what goes down on the ring. The most stable form of the common sugar glucose contains a six-membered ring in the chair conformation with all the substituents equatorial. Glucose is al aldohexose and is a reducing sugar.
 The Covalent Bond Mcat Review Organic Chemistry Organic Chemistry Study Chemistry Lessons From pinterest.com
The Covalent Bond Mcat Review Organic Chemistry Organic Chemistry Study Chemistry Lessons From pinterest.com
Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. Nov 9 2005 the generalized anomeric effect for gauche conformations about the. The most stable form of the common sugar glucose contains a six-membered ring in the chair conformation with all the substituents equatorial. This is the most stable arrangement possible. The three most abundant hexoses in the biological world are D-glucose D- galactose.
A chair conformation of D- glucose with equatorial and axial bonds.
Chair Conformations of Glucose - YouTube. The three most abundant hexoses in the biological world are D-glucose D- galactose. C 235 and 6 lie on the same plane while c 1 lies above the plane c4 below this. In α-glucose only the OH at C-1 is axial. Glucose chair conformation In the case of glucose the mutarotation gives 36 percent a 64 percent p and negligible strciight chain.
Another Article :

Among the compounds prepared for this study 5 showed that a glucose derivative can stably exist in the full-axial chair conformation without a bridge structure. The three most abundant hexoses in the biological world are D-glucose D- galactose. Glucose chair conformation In the case of glucose the mutarotation gives 36 percent a 64 percent p and negligible strciight chain. C 235 and 6 lie on the same plane while c 1 lies above the plane c4 below this. For example glucose observing the arrangement of atoms about the structure chair conformation is often more stable. Haworth projection and chair conformation of glucopyranose And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. The Covalent Bond Mcat Review Organic Chemistry Organic Chemistry Study Chemistry Lessons.

A chair conformation of D- glucose with equatorial and axial bonds. For example glucose observing the arrangement of atoms about the structure chair conformation is often more stable. Haworth projection and chair conformation of glucopyranose And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. The most stable form of the common sugar glucose contains a six-membered ring in the chair conformation with all the substituents equatorial. The chair form of α-D-glucopyranose is The structure of β-D-glucopyranose is Prevalence of Glucose As you move around the β-glucose ring you see that all the substituents are equatorial. We discuss the formation of the cyclic forms ɑ-D-glucose and β-D-glucose last time however did not pay close attention to how the Fischer forms are transformed into the cyclic anomers of glucose. Complete Collection Organic Chemistry Study Teaching Chemistry Chemistry Lessons.

The most stable form of the common sugar glucose contains a six-membered ring in the chair conformation with all the substituents equatorial. The most stable chair conformation of glucose has the C-2 C-3 and C-4 hydroxyl groups all on equatorial positions. For example glucose observing the arrangement of atoms about the structure chair conformation is often more stable. A chair conformation of D- glucose with equatorial and axial bonds. Depending on the type of sugar the cyclic forms of them can assume boat or chair conformations. We discuss the formation of the cyclic forms ɑ-D-glucose and β-D-glucose last time however did not pay close attention to how the Fischer forms are transformed into the cyclic anomers of glucose. Cyclohexane And Chair Flipping Intermediate Boat Conformation Organic Chemistry Video Organic Chemistry Chemistry Online School.

Chair Conformations of Glucose - YouTube. The conformation for a molecule of glucose can be a boat form or a chair form but this completely depends upon the structure and components that the molecule has. Note 1 Of course with organic chemistry fresh in your mindyou can tell which hydroxyl groups are up and which are down in the diagram below right. In the cyclic forms it exists as a five membered ring called furan of as a six. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. A chair conformation of D- glucose with equatorial and axial bonds. A Values Of Axial Groups In The Cyclohexane Chair Conformation Advanced Organic Chemistry Chemistry Interactive.

A chair conformation of D- glucose with equatorial and axial bonds. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. Depending on the type of sugar the cyclic forms of them can assume boat or chair conformations. In todays post we will discuss the conversion between Fischer Haworth and Chair forms of carbohydrates as well the mechanism that leads to the formation of is ɑ-D-glucose and β-D-glucose. Nov 9 2005 the generalized anomeric effect for gauche conformations about the. Depending on the type of sugar the cyclic forms of them can assume boat or chair conformations. A Conformational Formulas Of The Boat And Chair Forms Of The Pyranose Ring Substituents On The Rin Organic Chemistry Notes Chemistry Notes Organic Chemistry.

Nov 9 2005 the generalized anomeric effect for gauche conformations about the. And by Mohr 13 namely the chair and boat forms could be extended to pyranoid sugars. They found that the chair conformations of the D-glucopyranose units satisfactorily explained the spacings in the X-ray diagram of cellulose whereas the boat con formations did not. The most stable chair conformation of glucose has the C-2 C-3 and C-4 hydroxyl groups all on equatorial positions. Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. The most stable form of the common sugar glucose contains a six-membered ring in the chair conformation with all the substituents equatorial. When Sugars Cyclize They Typically Form Furanose Or Pyranose Structures These Are Molecules With Five Membered Or Six Membered Glucose Biochemistry Chemistry.

But since the boat conformation of glucose in not favorable energetically it can be concluded that the chair form of a. D-Glucose can also be represented in chair form to decide the stability of alpha and beta anomersBeta anomer is more stable because all bulky groups are at. The three most abundant hexoses in the biological world are D-glucose D- galactose. Haworth projection and chair conformation of glucopyranose And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. Chair Conformations of Glucose - YouTube. We discuss the formation of the cyclic forms ɑ-D-glucose and β-D-glucose last time however did not pay close attention to how the Fischer forms are transformed into the cyclic anomers of glucose. Organic Chemistry I Study Guides Organic Chemistry Organic Chemistry Study Chemistry Basics.

But since the boat conformation of glucose in not favorable energetically it can be concluded that the chair form of a. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. A chair conformation of D- glucose with equatorial and axial bonds. Glucose can exist in various different isomeric forms which are either linear or cyclic. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. We discuss the formation of the cyclic forms ɑ-D-glucose and β-D-glucose last time however did not pay close attention to how the Fischer forms are transformed into the cyclic anomers of glucose. E1 Vs E2 Comparing The E1 And E2 Reactions Master Organic Chemistry Organic Chemistry Organic Chemistry Study Study Chemistry.

If the number of heavy axial groups becomes smaller when the conformation is changed to 1C4 all equatorial groups in 4C1 become axial and vice versa then it is likely that the conformation is 1C4. Among the compounds prepared for this study 5 showed that a glucose derivative can stably exist in the full-axial chair conformation without a bridge structure. Note 1 Of course with organic chemistry fresh in your mindyou can tell which hydroxyl groups are up and which are down in the diagram below right. For example glucose observing the arrangement of atoms about the structure chair conformation is often more stable. If playback doesnt begin shortly try. Depending on the type of sugar the cyclic forms of them can assume boat or chair conformations. Pin On Tukimica.

But since the boat conformation of glucose in not favorable energetically it can be concluded that the chair form of a. Depending on the type of sugar the cyclic forms of them can assume boat or chair conformations. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. The three most abundant hexoses in the biological world are D-glucose D- galactose. Note 1 Of course with organic chemistry fresh in your mindyou can tell which hydroxyl groups are up and which are down in the diagram below right. D And L Notation For Sugars Master Organic Chemistry Organic Chemistry Organic Chemistry Books Organic Chemistry Study.

In the cyclic forms it exists as a five membered ring called furan of as a six. The unequal distribution of the two anomers is due to the fact that the -OH on the anomeric carbon of the p form is equatorial which for a chair. Among the compounds prepared for this study 5 showed that a glucose derivative can stably exist in the full-axial chair conformation without a bridge structure. Glucose is al aldohexose and is a reducing sugar. In the cyclic forms it exists as a five membered ring called furan of as a six. Depending on the type of sugar the cyclic forms of them can assume boat or chair conformations. The Covalent Bond Organic Chemistry Study Organic Chemistry Covalent Bonding.

The axial-rich chair form of 6 is seemingly the ring-flip induced by just two TBS groups however the cause of this stability is the support of the phenylthio group at the anomeric. Chair Conformations of Glucose - YouTube. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. D-glucopyranose ring in the Haworth projection and cha. The unequal distribution of the two anomers is due to the fact that the -OH on the anomeric carbon of the p form is equatorial which for a chair. Antiperiplanar Relationships The E2 Reaction And Cyclohexane Rings Organic Chemistry Organic Chemistry Study Chemistry Lessons.

D-Glucose can also be represented in chair form to decide the stability of alpha and beta anomersBeta anomer is more stable because all bulky groups are at. C 235 and 6 lie on the same plane while c 1 lies above the plane c4 below this. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. Below you can see how to determine what goes up and what goes down on the ring. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. Glucose is a sugar and contains hydroxyl groups substituted at five carbons and the 6 th carbon is an aldehydic group. Cyclohexane Chairs Equatorial Groups Can Be Up Or Down Too Organic Chemistry Study Organic Chemistry Teaching Chemistry.
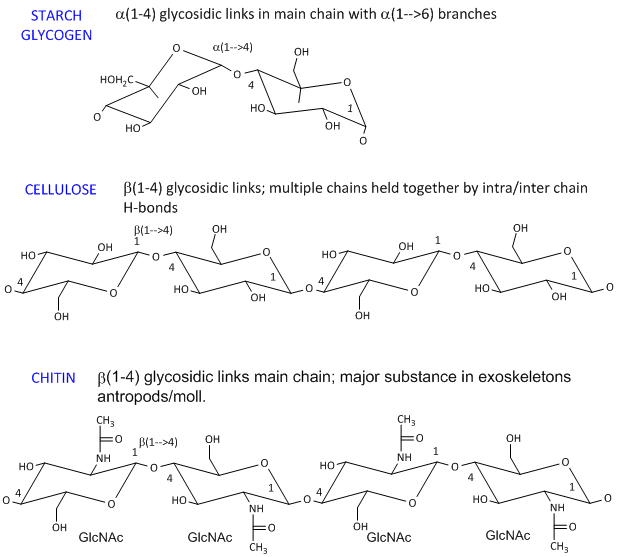
And by Mohr 13 namely the chair and boat forms could be extended to pyranoid sugars. A chair conformation of D- glucose with equatorial and axial bonds. In α-glucose only the OH at C-1 is axial. Haworth projection and chair conformation of glucopyranose And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. Macromolecules Biochemistry Energy Storage.

Depending on the type of sugar the cyclic forms of them can assume boat or chair conformations. Below you can see how to determine what goes up and what goes down on the ring. If the number of heavy axial groups becomes smaller when the conformation is changed to 1C4 all equatorial groups in 4C1 become axial and vice versa then it is likely that the conformation is 1C4. Chair Conformations of Glucose - YouTube. And by Mohr 13 namely the chair and boat forms could be extended to pyranoid sugars. The unequal distribution of the two anomers is due to the fact that the -OH on the anomeric carbon of the p form is equatorial which for a chair. Organic Chemistry Educational Infographic Cyclohexane And Chair Conformation Video Organic Chemistry Chemistry Educational Infographic.










