chair conformation energy Thus it is the most popular. Twist-boat and boat conformations are higher in energy than chair and are similar in energy levels although.
Chair Conformation Energy, The higher energy chair conformation contains two axial methyl groups. The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain.
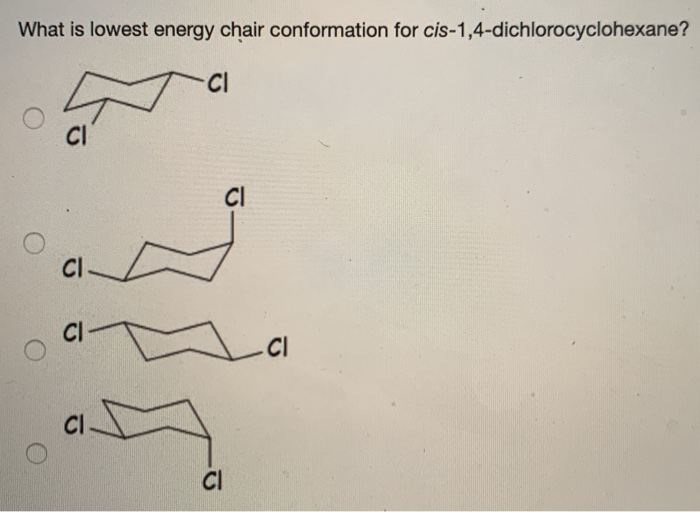 Solved What Is Lowest Energy Chair Conformation For Chegg Com From chegg.com
Solved What Is Lowest Energy Chair Conformation For Chegg Com From chegg.com
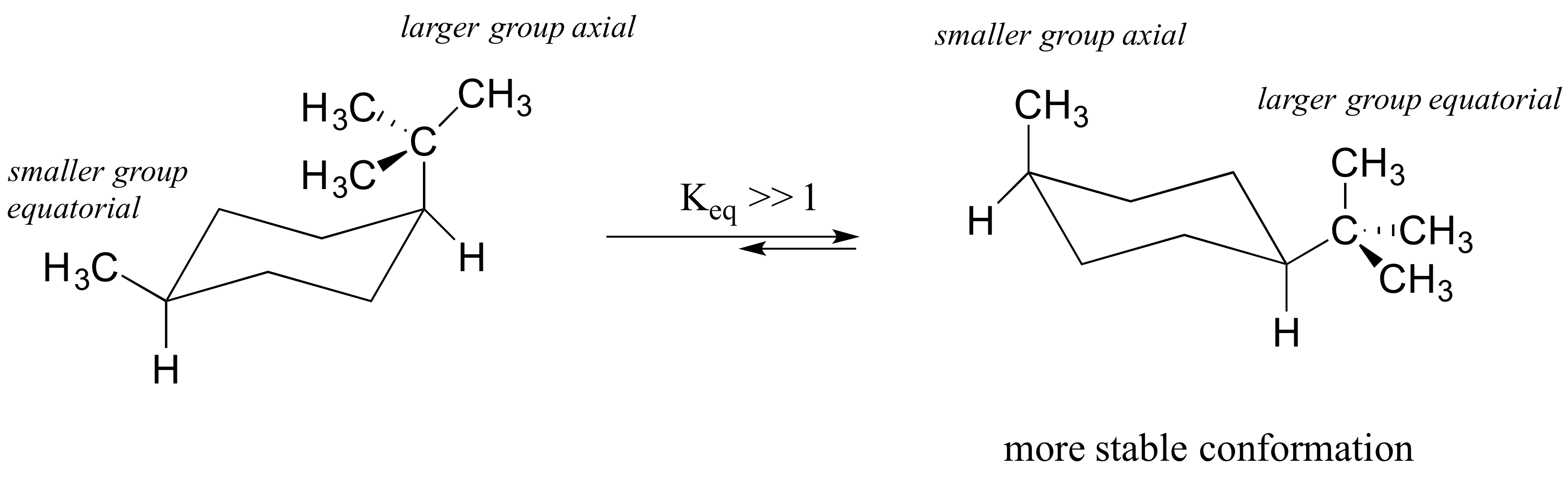
The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. We can see in this energy diagram that if we want to pass from the twist-boat to the chair we will need to increase the energy about 22 kJmol before reaching the peak at 43 kJmol where we will start to descend towards the chair conformation at the very bottom at 0 kJmol. However since this conformation is 55 kcalmol higher in energy than the chair conformation it is not a highly populated stateAgain most of the strain energy results from bonds which are eclipsed or partially eclipsed ie from torsional strain. The terms chair conformation and boat conformation come under organic chemistry and they are mainly applicable to cyclohexane. The second stage may be described as a very easy transformation of one twist-boat conformation to another via boat almost without any changes in energy.
We can see in this energy diagram that if we want to pass from the twist-boat to the chair we will need to increase the energy about 22 kJmol before reaching the peak at 43 kJmol where we will start to descend towards the chair conformation at the very bottom at 0 kJmol.
The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1. A The two chair conformations are equal in energy. -the presence of 13-di axial interactions causes the chair conformation to be higher in energy when the substituent is in an axial position-When the substituent is in an equal atorial position these 13-diaxial gauche interactions are absent no gauche interaction H H H H H CH no gauche H H interaction 1-1 H H H-the equilibrium between the two Chair conformations will generally favor the conformation with the. However keep in mind that the geometry of the cyclohexane needs to be. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation.
Another Article :

However keep in mind that the geometry of the cyclohexane needs to be. The lowest energy conformation is the chair conformation. The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. In the first conformer we have two chlorines in axial positions so the total steric strain is. These are two different structures in which the cyclohexane molecule can exist but they have different stabilities depending on the energy. A The two chair conformations are equal in energy. Energy Profile Of Cyclohexene Conformations Chemistry Stack Exchange.

A The two chair conformations are equal in energy. The first stage involves the transition from an equilibrium half-chair conformation to a twist-boat conformation with a significant increase in energy. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. The higher energy chair conformation contains two axial methyl groups. Here we will learn how to draw cyclohexane chairs how to flip them the differ. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. Rules Of Thumb Rots For Chair Conformations And Substituent Stability Teach The Mechanism.
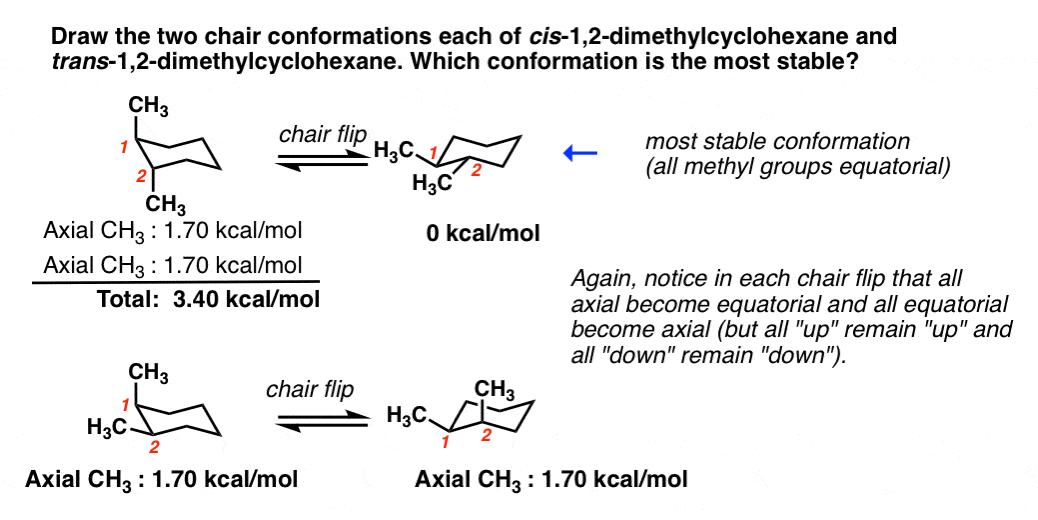
Thus it is the most popular. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. The lower energy chair conformation contains two axial methyl groups. The lower energy chair conformation contains two axial methyl groups. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
Twist-boat and boat conformations are higher in energy than chair and are similar in energy levels although. -the presence of 13-di axial interactions causes the chair conformation to be higher in energy when the substituent is in an axial position-When the substituent is in an equal atorial position these 13-diaxial gauche interactions are absent no gauche interaction H H H H H CH no gauche H H interaction 1-1 H H H-the equilibrium between the two Chair conformations will generally favor the conformation with the. The higher energy chair conformation contains two axial methyl groups. The lower energy chair conformation contains two axial methyl groups. The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. The terms chair conformation and boat conformation come under organic chemistry and they are mainly applicable to cyclohexane. Answer In Organic Chemistry For Rakesh Kumar 104929.

The second stage may be described as a very easy transformation of one twist-boat conformation to another via boat almost without any changes in energy. The higher energy chair conformation contains two axial methyl groups. The lower energy chair conformation contains two axial methyl groups. The first stage involves the transition from an equilibrium half-chair conformation to a twist-boat conformation with a significant increase in energy. The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. The lowest energy conformation is the chair conformation. Solution Draw The Lowest Energy Chair Co Organic Chem.
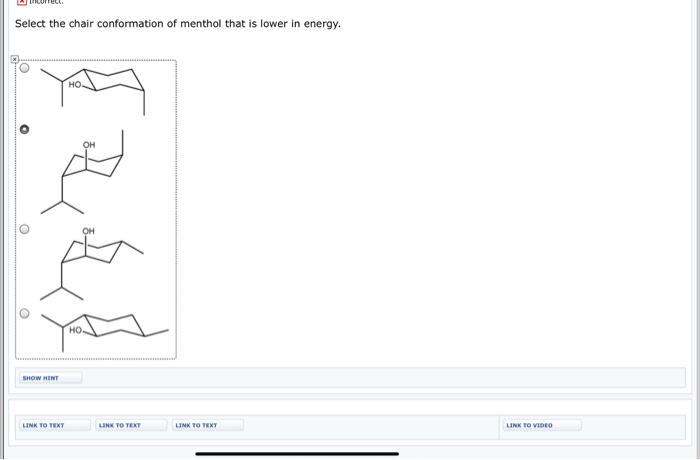
This is a multistep process so here Im going to walk you through it from scratch. -the presence of 13-di axial interactions causes the chair conformation to be higher in energy when the substituent is in an axial position-When the substituent is in an equal atorial position these 13-diaxial gauche interactions are absent no gauche interaction H H H H H CH no gauche H H interaction 1-1 H H H-the equilibrium between the two Chair conformations will generally favor the conformation with the. The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. In the first conformer we have two chlorines in axial positions so the total steric strain is. This means that the chair conformation is the structure that is observable. For each chair conformer add the energy of all the groups on axial position. Solved Morrect Select The Chair Conformation Of Menthol That Chegg Com.
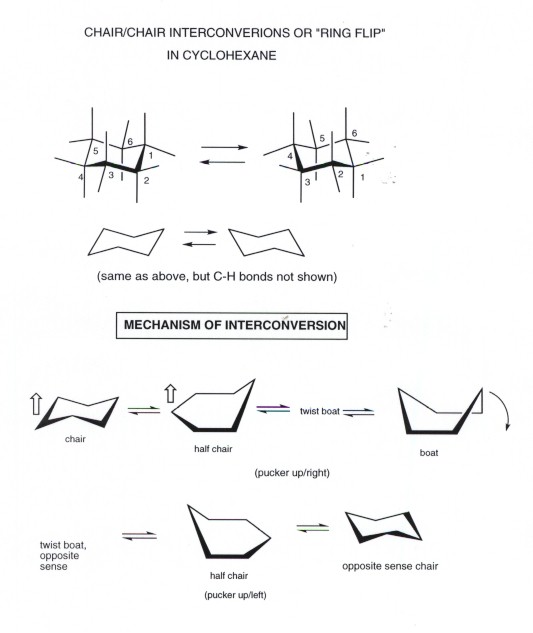
This is true for 1R-33-dichlorocyclohexanol. The most important is the chair conformation. Always place the largesthighest priority group in the equatorial position. 2222 44 kJ. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. Twist-boat and boat conformations are higher in energy than chair and are similar in energy levels although. Cyclohexane Conformational Analysis.

The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. The first stage involves the transition from an equilibrium half-chair conformation to a twist-boat conformation with a significant increase in energy. Draw the second chair conformation ring-flip-check this post if not sure. The higher energy chair conformation contains two axial methyl groups. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1. The terms chair conformation and boat conformation come under organic chemistry and they are mainly applicable to cyclohexane. Solved A Which Has The Highest Energy Diaxial Chair Chegg Com.
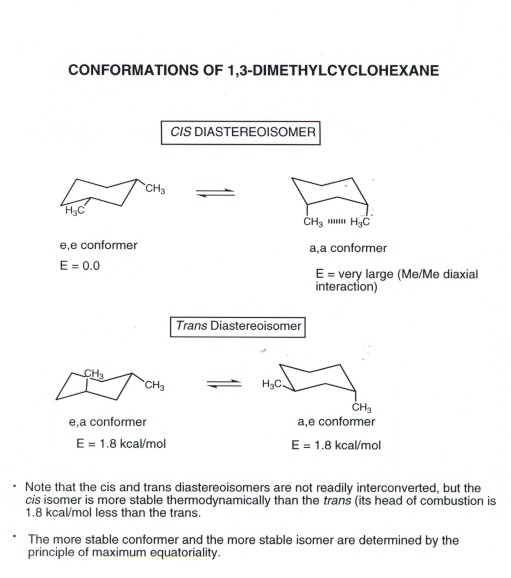
These are two different structures in which the cyclohexane molecule can exist but they have different stabilities depending on the energy. The higher energy chair conformation contains two axial methyl groups. A The two chair conformations are equal in energy. The most important is the chair conformation. Always place the largesthighest priority group in the equatorial position. Cyclohexane makes weird shapes. Cyclohexane Conformational Analysis.

This is a multistep process so here Im going to walk you through it from scratch. These are two different structures in which the cyclohexane molecule can exist but they have different stabilities depending on the energy. A The two chair conformations are equal in energy. The first stage involves the transition from an equilibrium half-chair conformation to a twist-boat conformation with a significant increase in energy. The second stage may be described as a very easy transformation of one twist-boat conformation to another via boat almost without any changes in energy. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
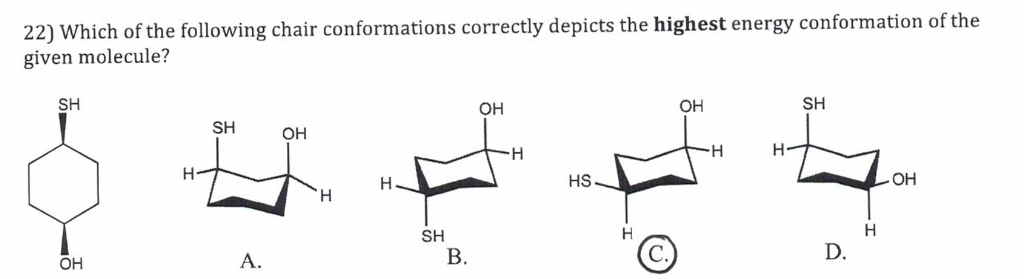
In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Always place the largesthighest priority group in the equatorial position. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. This means that the chair conformation is the structure that is observable. Solved 22 Which Of The Following Chair Conformations Chegg Com.
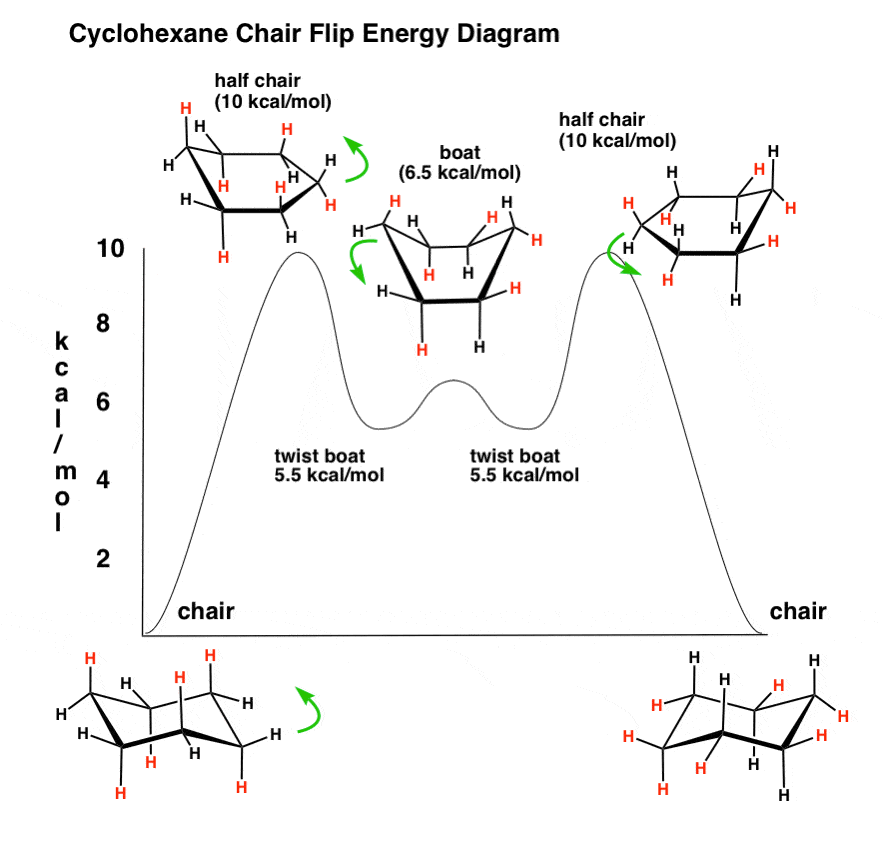
The lowest energy conformation is the chair conformation. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1. Cyclohexane makes weird shapes. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. This is true for 1R-33-dichlorocyclohexanol. The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. Why Is The Cyclohexane Ring Free Of Strain.
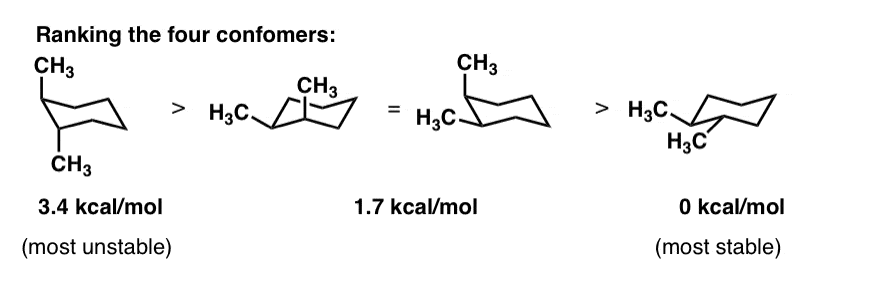
The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. The terms chair conformation and boat conformation come under organic chemistry and they are mainly applicable to cyclohexane. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. We can see in this energy diagram that if we want to pass from the twist-boat to the chair we will need to increase the energy about 22 kJmol before reaching the peak at 43 kJmol where we will start to descend towards the chair conformation at the very bottom at 0 kJmol. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.

The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. However since this conformation is 55 kcalmol higher in energy than the chair conformation it is not a highly populated stateAgain most of the strain energy results from bonds which are eclipsed or partially eclipsed ie from torsional strain. A The two chair conformations are equal in energy. The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. The lower energy chair conformation contains two axial methyl groups. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.
The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. For each chair conformer add the energy of all the groups on axial position. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. This is a multistep process so here Im going to walk you through it from scratch. The lowest energy conformation is the chair conformation. Dzhd Liniya Na Sajta Drebna Riba Most Stable Chair Conformation Tricomfireprotection Com.











