ahu design in pharma If the air extracted from the hood is not accounted for in the HVAC calculations there might not be enough air pushed into the room to maintain positive pressurisation. HVAC Design for Pharmaceutical Facilities In pharmaceutical manufacturing how space conditions impact the product being made is of primary importance.
Ahu Design In Pharma, Ahu validation in pharma pdf These guidelines mainly focus on recommendations for HVAC systems used in. If the air extracted from the hood is not accounted for in the HVAC calculations there might not be enough air pushed into the room to maintain positive pressurisation. Ventilation with 100 fresh air no air re-circulation W Washer optional Central Air Handling Unit Production Rooms Exhaust Unit.
 Hvac System In Pharmaceutical Industry From pharmaengineers.com
Hvac System In Pharmaceutical Industry From pharmaengineers.com
AHU -Air handling units Dampers Coils and Valves Fans Distribution ducts and terminal boxes Pumps and Plumbing Control devices and control loops Unitary equipment. Ahu validation in pharma pdf These guidelines mainly focus on recommendations for HVAC systems used in. Janki Singh is experienced in Pharmaceuticals author and founder of Pharma Beginners an ultimate pharmaceutical blogging platform. Basic components of HVAC. Average velocity and subsequent air changes per hour shall be within design qualifications.
Deflection shall be measured within an uncertainty of 05 mm whilst the air handling unit is operating at its normal design condition.
An air handler is usually a large metal box containing a blower heating or cooling elements filter racks or chambers sound attenuators and dampers. An air handler is usually a large metal box containing a blower heating or cooling elements filter racks or chambers sound attenuators and dampers. This fresh air will need to be conditioned temperature and relative humidity and the process to achieve that requires a larger air make-up or air handling unit AHU. Dust control during AHU design in Pharma Company. Air conditioning has changed over the years the HVAC system is used to control the environment in the manufacturing as well as the storage area of the pharmaceutical facility.
Another Article :

HVAC Design for Pharmaceutical Facilities In pharmaceutical manufacturing how space conditions impact the product being made is of primary importance. AHU -Air handling units Dampers Coils and Valves Fans Distribution ducts and terminal boxes Pumps and Plumbing Control devices and control loops Unitary equipment. The diagram below shows the components of our system. Dust control in Pharma Company in measure challenge Wherever possible dust or vapour contamination should be removed at source. AHU control and operation sequences especially for typical in-patient floors. As close as possible to the point where the dust is generated should be employed. Air Lock Hvac System Of Pharmaceutical Industry English Youtube.
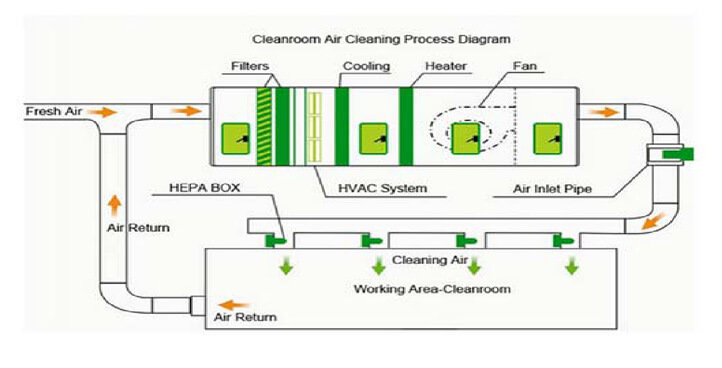
An air handler or air handling unit often abbreviated to AHU is a device used to condition and circulate air as part of a heating ventilating and air-conditioning HVAC system. The AHU is the air handler or handling unit. An air handler is usually a large metal box containing a blower heating or cooling elements filter racks or chambers sound attenuators and dampers. In this paper the necessary classifications for clean air air handling unit and its different components are discussed. Air conditioning has changed over the years the HVAC system is used to control the environment in the manufacturing as well as the storage area of the pharmaceutical facility. Basic components of HVAC. Clean Room Ahu Design Ksa G Com.

Your competent contact person regarding this topic is. 125 126 Temperature relative humidity and ventilation should be appropriate and should not 127 adversely affect the quality of pharmaceutical products during their manufacture and storage. This is the classification we usually specify for our AHU. Pharmacy HVAC Design Reference USP 795 - Pharmaceutical Compounding - Non-sterile preparations 2015 USP 797 - Pharmaceutical Compounding - Sterile preparations. Air Handling Unit AHU is a device used to condition and circulate air as. Air Handling Unit 2. Air Handling Unit Cdr Cool Point.
Pharmaceutical industry guarantees successful designing and manufacturing of optimal HVAC solution. The AHU is the air handler or handling unit. Dust control in Pharma Company in measure challenge Wherever possible dust or vapour contamination should be removed at source. Pressure drops all through the duct system in Pharma Industry. Average velocity and subsequent air changes per hour shall be within design qualifications. The diagram below shows the components of our system. Air Handling System In Pharmaceutical Manufacturing Ppt Download.
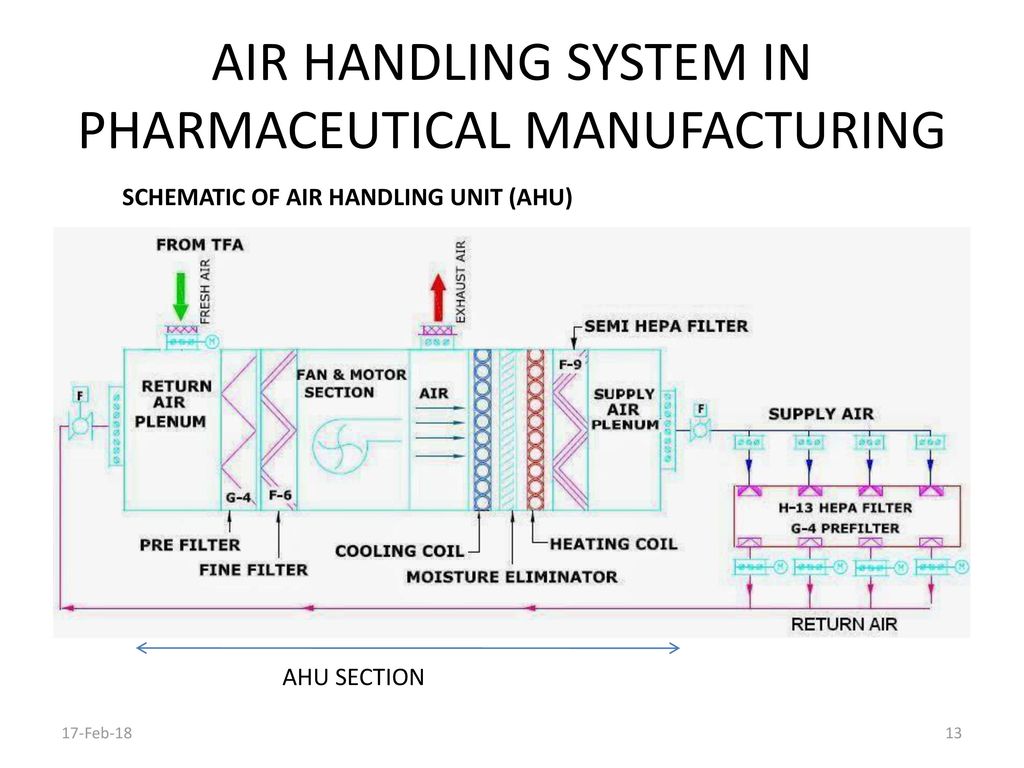
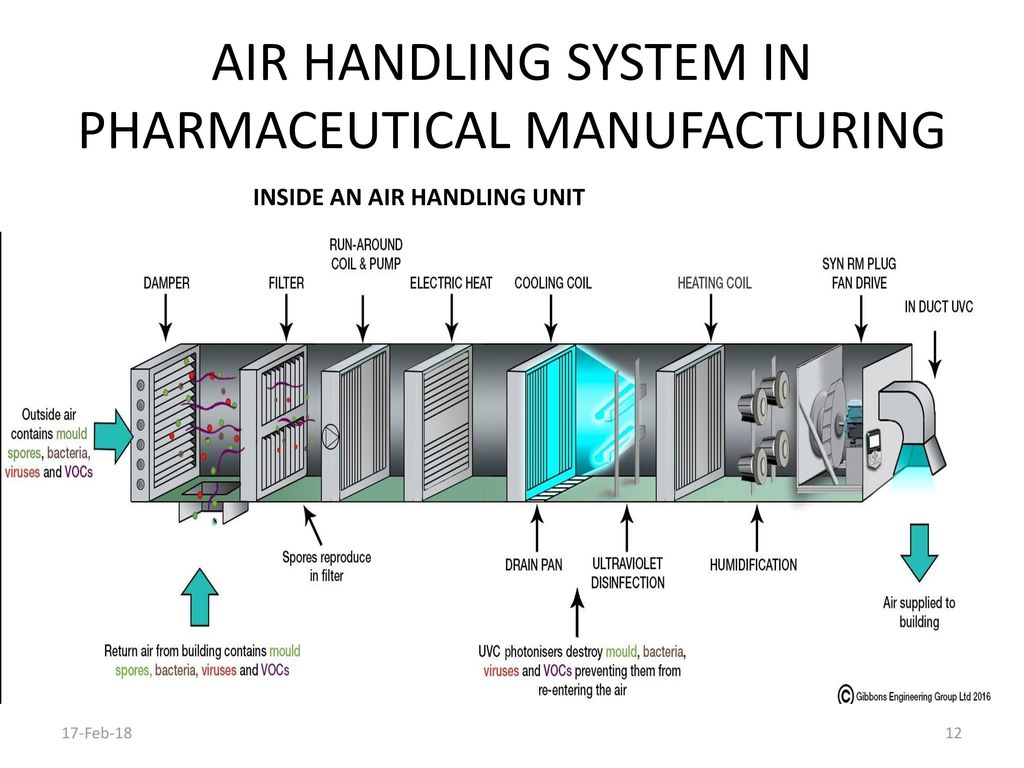
Conventional air handling units consist of filters coils and fans in a metal casing with an insulation liner applied to the inside of the casing. The objective of this protocol is to provide an outline for the qualification of the HVAC system and to establish documentary evidence to demonstrate that the Air Handling Units AHUs are qualified to perform well within the predetermined acceptance criteria of performance as per guideline outlined in this protocol. Whenever air encounters a filter coil heat exchanger registers grilles balancing dampers and the ducts themselves it loses pressure. Upon submission of your air handling unit requirements either to our experienced technical sales engineers who cover the length and breadth of the UK or direct in to head office initial design at tender stage is carried out by our experienced team of estimators and application engineers. An air handler is usually a large metal box containing a blower heating or cooling elements filter racks or chambers sound attenuators and dampers. The diagram below shows the components of our system. Air Handling System In Pharmaceutical Manufacturing Ppt Download.

If the air extracted from the hood is not accounted for in the HVAC calculations there might not be enough air pushed into the room to maintain positive pressurisation. Practices GMP requirements for HVAC systems for facilities for the manufacture of solid place at the conclusion of project construction but prior to validation. Dust control in Pharma Company in measure challenge Wherever possible dust or vapour contamination should be removed at source. This document aims to give guidance to pharmaceutical manufacturers. Fan coils perimeter radiation unit ventilators unit heaters etc. Air conditioning has changed over the years the HVAC system is used to control the environment in the manufacturing as well as the storage area of the pharmaceutical facility. Hvac System.

In this paper the necessary classifications for clean air air handling unit and its different components are discussed. Air Handling Unit AHU is a device used to condition and circulate air as. 30 Objective HVAC System Qualification Protocol. AHU is of recirculated type and made from double skin panels filled with PUF insulation density of 48 kgm3 and assembled as a closed box with Aluminum profiles. Extreme fluctuations in temperature and moisture stress the material to a considerable extent. The AHU is the air handler or handling unit. Air Handling Unit Ahu In Pharmaceuticals Youtube.
Using AHU return duct would also warrant replacement of ducting. Janki Singh is experienced in Pharmaceuticals author and founder of Pharma Beginners an ultimate pharmaceutical blogging platform. Pharmacy HVAC Design Reference USP 795 - Pharmaceutical Compounding - Non-sterile preparations 2015 USP 797 - Pharmaceutical Compounding - Sterile preparations. HVAC systems Heating Ventilation and Air Conditioning systems are the integral part of environmental control system design. Average velocity and subsequent air changes per hour shall be within design qualifications. AHU Prefilter Final filter 21 Positioning of filters 31. What Is The Difference Between Tfa And Ahu Hvac Quora.
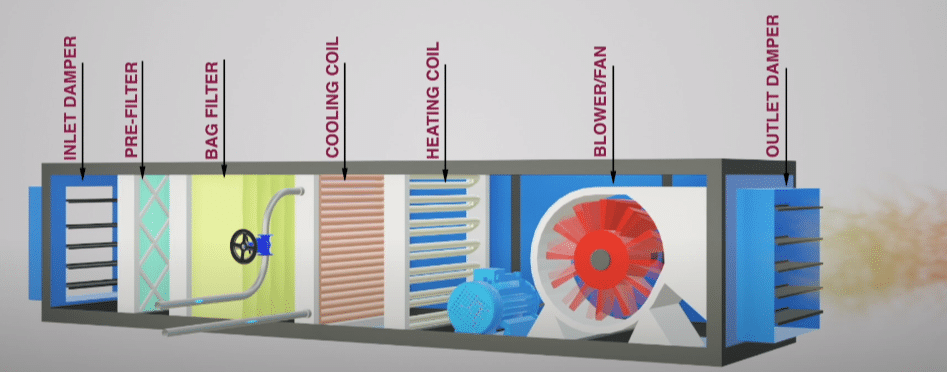
Our AHU design process. Whenever air encounters a filter coil heat exchanger registers grilles balancing dampers and the ducts themselves it loses pressure. 1041722379-17641000202 PPT Presentation Open Access Advanced Techniques in A Biology Medicine. HVAC Design for Pharmaceutical Facilities In pharmaceutical manufacturing how space conditions impact the product being made is of primary importance. The objective of this protocol is to provide an outline for the qualification of the HVAC system and to establish documentary evidence to demonstrate that the Air Handling Units AHUs are qualified to perform well within the predetermined acceptance criteria of performance as per guideline outlined in this protocol. For example referring to the following figure XX measured for span RS XX is measured for span PQ. Pharmaceutical Ahu And Hvac Components Pharmaguddu.

The pharmaceutical facilities are closely supervised by the US. 30 Objective HVAC System Qualification Protocol. Huber Ranner offer the AHU you need. Dust control during AHU design in Pharma Company. Design stage of a pharmaceutical manufacturing plant. During the project construction but prior to validation. Hvac System In Pharmaceutical Industry.
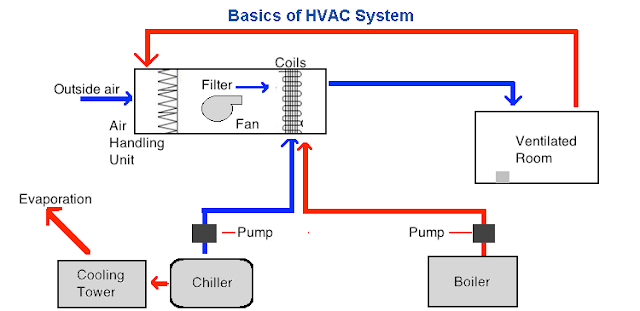
Janki Singh is experienced in Pharmaceuticals author and founder of Pharma Beginners an ultimate pharmaceutical blogging platform. Air distribution Dust network Insulator Dampersvalves Return lower grills. Also for the range hygienics pharma Huber Ranner can offer a multiplicity of solutions and products. Deflection shall be measured within an uncertainty of 05 mm whilst the air handling unit is operating at its normal design condition. Air Handling Unit 2. Air Handling Unit AHU is a device used to condition and circulate air as. Basics Of Hvac System Pharmaceutical Guidelines.

Air Handling Unit 2. Food and drug administration FDA which requires manufacturing companies to conform to cGMP current Good Manufacturing Practices. Pressure drops all through the duct system in Pharma Industry. 125 126 Temperature relative humidity and ventilation should be appropriate and should not 127 adversely affect the quality of pharmaceutical products during their manufacture and storage. The objective of this protocol is to provide an outline for the qualification of the HVAC system and to establish documentary evidence to demonstrate that the Air Handling Units AHUs are qualified to perform well within the predetermined acceptance criteria of performance as per guideline outlined in this protocol. As close as possible to the point where the dust is generated should be employed. Air Handling System In Pharmaceutical Manufacturing Ppt Download.
Huber Ranner offer the AHU you need. Food and drug administration FDA which requires manufacturing companies to conform to cGMP current Good Manufacturing Practices. Extreme fluctuations in temperature and moisture stress the material to a considerable extent. Also for the range hygienics pharma Huber Ranner can offer a multiplicity of solutions and products. 1041722379-17641000202 PPT Presentation Open Access Advanced Techniques in A Biology Medicine. Ahu validation in pharma pdf These guidelines mainly focus on recommendations for HVAC systems used in. Asiapharmaceutics Info.
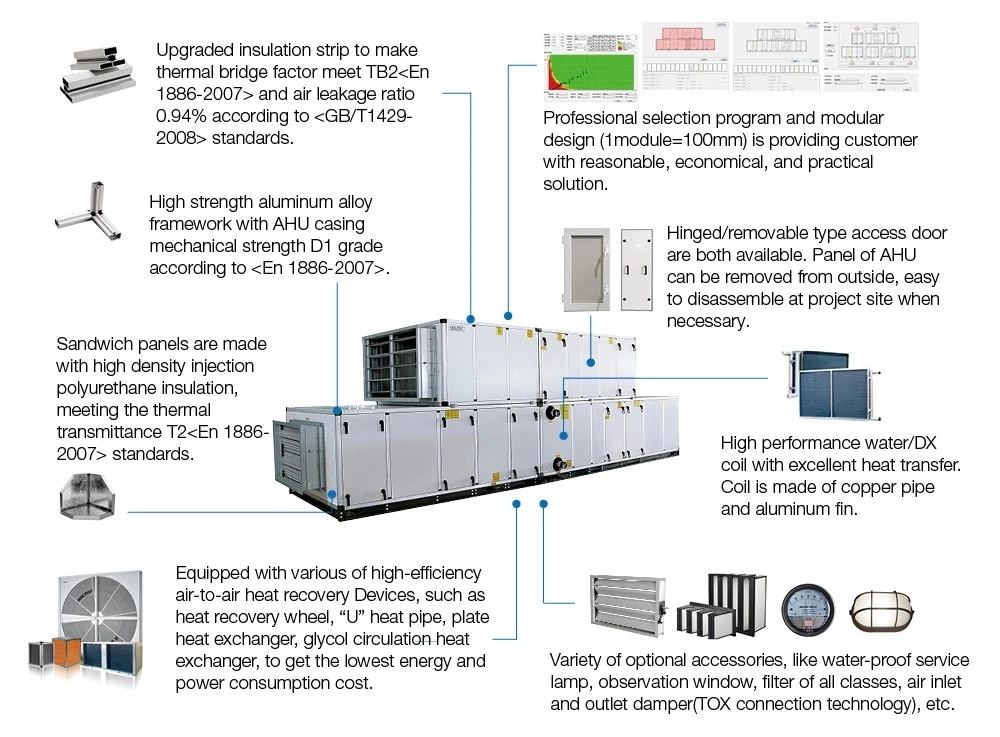
30 Objective HVAC System Qualification Protocol. Fan coils perimeter radiation unit ventilators unit heaters etc. Spot ventilation or capture hoods may be used as appropriate. The pharmaceutical facilities are closely supervised by the US. Conventional air handling units consist of filters coils and fans in a metal casing with an insulation liner applied to the inside of the casing. Also for the range hygienics pharma Huber Ranner can offer a multiplicity of solutions and products. Pharmaceutical Workshop Clean Room Hvac System Purification Air Handling Unit Temperature And Humidifty Control Ahu Buy Rooftop Air Handling Unit Air Handling Unit Pharmaceutical Workshop Product On Alibaba Com.

1041722379-17641000202 PPT Presentation Open Access Advanced Techniques in A Biology Medicine. Whenever air encounters a filter coil heat exchanger registers grilles balancing dampers and the ducts themselves it loses pressure. Dust control in Pharma Company in measure challenge Wherever possible dust or vapour contamination should be removed at source. Food and drug administration FDA which requires manufacturing companies to conform to cGMP current Good Manufacturing Practices. 125 126 Temperature relative humidity and ventilation should be appropriate and should not 127 adversely affect the quality of pharmaceutical products during their manufacture and storage. Janki Singh is experienced in Pharmaceuticals author and founder of Pharma Beginners an ultimate pharmaceutical blogging platform. N Tech Stainless Steel Air Handling Unit System Pharma Project For Industrial Use Capacity 1000 Cfm To 40000 Cfm Id 9507057333.











