chair conformation with oxygen There is however one major effect which is much stronger in tetrahydropyrans than in cyclohexanes and is induced by the presence of oxygen lone pairs which replace some C-H bonds. This cyclic structure is called the pyranose ring form of the sugar.
Chair Conformation With Oxygen, As one might expect the tetrahydropyran ring adopts a chair conformation and is largely similar to cyclohexane. Oxygens equatorial lone pairs are parallel with nothing but bonds in the ring so the oxygens axial lone pair is the only one that can help stabilize the molecule and it can only do this when the Cl is axial. Each substituent is labeled UP or DOWN and then placed in the appropriate position on the chair conformation.
 The Haworth Projection Master Organic Chemistry From masterorganicchemistry.com
The Haworth Projection Master Organic Chemistry From masterorganicchemistry.com
For example the energy difference of the axial ethyl cyclohexane with the equatorial. Explaining how A-Values are related to cyclohexane flip energy. Cyclohexane and the Chair Structure. O H H H H H H H H H H O. Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator.
Note how the oxygen is placed on the upper rear-right corner.
Calculating Flip Energy. All carbon centers are equivalent. Substituents attached to the ring lie above or below the plane. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. In glucose all the hydroxy groups are in the equatorial position.
Another Article :
Note how the oxygen is placed on the upper rear-right corner. The larger the group the higher the energy difference. Cyclohexane and the Chair Structure. This energy difference is known as the A value and it varies depending on the axial group. 4 points CH3 Least stable chair Most stable chair CH3G Ð36 kcalmol Question 3 is continued on the next page. The symmetry is D3d. The Haworth Projection Master Organic Chemistry.

C-1 is easily identified because it is the hemiacetal carbon-the only carbon bonded to two oxygen atoms. O H H H H H H H H H H O. Oxygen - hexoses O HO HO H OH H OH H H CH2OH H O HO HO H H OH OH H H CH2OH H OH HO HO H H OH H H CH2OH H O α-D-glucose 36 002 β-D-glucose 64 all equatorial all equatorial except anomeric carbon including anomeric carbon Nitrogen - piperidines. Because of the hydroxyl groups and oxygen atoms there are 38 distinct conformations 2 chairs 6 boats 6 skew-boats 12 half-chairs and 12 envelopes. Chair conformations are easily drawn by recognizing the differences between the sugar in question and glucose. Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator. The Haworth Projection Organic Chemistry Jempol Kimia.

What is this position and what is the energy level kJmol. Calculating Flip Energy. There is however one major effect which is much stronger in tetrahydropyrans than in cyclohexanes and is induced by the presence of oxygen lone pairs which replace some C-H bonds. What is this position and what is the energy level kJmol. Only the axial conformation benefits from the stabilization and this is the origin of the anomeric effect. We can now move arond the exterior oxygen molecules to find in which postition equatorial or axial the glucose reaches its lowest energy level. 1 3 Diaxial Interactions And A Value For Cyclohexanes Chemistry Steps.
With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. If you switch out a carbon atom for an oxygen atom and you are set to study the pyranoses of biochemistry eg. All carbon centers are equivalent. For example the energy difference of the axial ethyl cyclohexane with the equatorial. That forms between the oxygen atom on C-5 and the hemiacetal carbon atom C-1 is usually shown by using a box in the Fischer projection. Ive found that some of the best depictions of six-member-ring conformations come from pyranose sugars. Heterocyclics.
What is this position and what is the energy level kJmol. The most stable chair conformation of cis-13-cyclohexanediol has both hydroxyl groups in axial positions. Explain with a drawing why this is the case 2 marks. A Newman projection of a chair conformation of cyclohexane clearly shows that torsional strain is minimized since all groups are staggered. When the cyclic hemiacetal forms the C-1 carbon atom becomes a new stereogenic center and can have either an R- or an S-configuration. It looks like trans-decalin. The Haworth Projection Master Organic Chemistry.
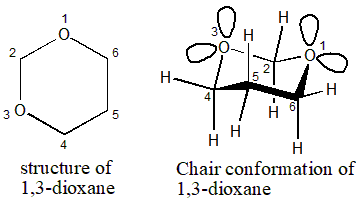
For aldoses having high stability in one chair conformation the rates of oxidation of. This first conformation is called the chair conformation. The symmetry is D3d. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. When the cyclic hemiacetal forms the C-1 carbon atom becomes a new stereogenic center and can have either an R- or an S-configuration. If you switch out a carbon atom for an oxygen atom and you are set to study the pyranoses of biochemistry eg. Solved The Most Stable Chair Conformation Of 1 3 Dioxane 5 Ol Has Chegg Com.

Two macrocyclic molecules incorporate four water molecules and one of the two ketonic carbonyl oxygen atoms of each macrocyclic molecule participates in construction of a twelve-membered ring composed of alternatively sequenced oxygen and hydrogen atoms ie ketone-hybridized cyclic oxygen water hexamer with a chair conformation in the asymmetric unit of Pccn space group Figure 3. A Newman projection of a chair conformation of cyclohexane clearly shows that torsional strain is minimized since all groups are staggered. There is however one major effect which is much stronger in tetrahydropyrans than in cyclohexanes and is induced by the presence of oxygen lone pairs which replace some C-H bonds. Presumably this conformation is stabilized by resonance involving the oxygen atom of the ring. Explaining how A-Values are related to cyclohexane flip energy. In both chair conformations is so low that they probably exist in a variety of conformations the rates of ox idation of the anomers show little difference and no particular correlation with the angular position of the Cl-hydroxyl group. Converting Fischer Haworth And Chair Forms Of Carbohydrates Chemistry Steps.

Ive found that some of the best depictions of six-member-ring conformations come from pyranose sugars. Two macrocyclic molecules incorporate four water molecules and one of the two ketonic carbonyl oxygen atoms of each macrocyclic molecule participates in construction of a twelve-membered ring composed of alternatively sequenced oxygen and hydrogen atoms ie ketone-hybridized cyclic oxygen water hexamer with a chair conformation in the asymmetric unit of Pccn space group Figure 3. B trans-14-Dimethylcyclohexane shown below also exists in two different chair conformations one of which is 36 kcalmol more stable than the other. In each of the boxes below draw in methyl Me groups in the appropriate positions. Thus the energy barrier for ring flipping is about 10 kcalmol in each case. The chair structure of cyclohexane is considered to be the perfect conformation. One Chair Conformation Of The Sugar Galact Clutch Prep.
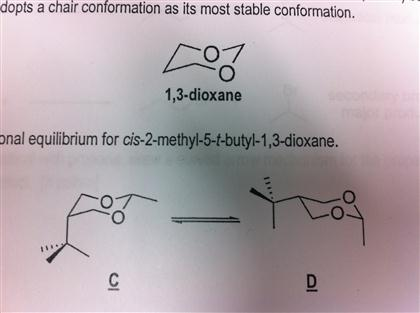
That forms between the oxygen atom on C-5 and the hemiacetal carbon atom C-1 is usually shown by using a box in the Fischer projection. In each of the boxes below draw in methyl Me groups in the appropriate positions. Note how the oxygen is placed on the upper rear-right corner. O H H H H H H H H H H O. Draw the compound below in an all chair conformation with an intramo ecular hydrogen bond AS A DOTTED INE between the a cohol proton 10 and the oxygen of the ring 5 points Flat-Ring Chairs clearly showing intramolecular H-Bond between 10 and 1. Because of the hydroxyl groups and oxygen atoms there are 38 distinct conformations 2 chairs 6 boats 6 skew-boats 12 half-chairs and 12 envelopes. Solved 1 3 Dioxane Shown Above Is A Heterocycle Where Two Chegg Com.

First draw the skeleton of a chair and number the carbons as follows. C-1 is easily identified because it is the hemiacetal carbon-the only carbon bonded to two oxygen atoms. This energy difference is known as the A value and it varies depending on the axial group. The next step is to draw all substituents on the chair. When the cyclic hemiacetal forms the C-1 carbon atom becomes a new stereogenic center and can have either an R- or an S-configuration. Two macrocyclic molecules incorporate four water molecules and one of the two ketonic carbonyl oxygen atoms of each macrocyclic molecule participates in construction of a twelve-membered ring composed of alternatively sequenced oxygen and hydrogen atoms ie ketone-hybridized cyclic oxygen water hexamer with a chair conformation in the asymmetric unit of Pccn space group Figure 3. Solution Draw The Most Stable Chair Confo Clutch Prep.

Substituents attached to the ring lie above or below the plane. All carbon centers are equivalent. The larger the group the higher the energy difference. Special Considerations with Cyclohexane Notice how the 2D and 3D representations of cyclohexane are quite different 2D - hexagon 3D - chair 5 of 7 0 Addition of the 6th carbon atom allow for more flexibility within the molecule in order to adopt different conformations WITHOUT breaking any bonds O 0 This is known as a chair conformation with the optimized bond angle near that of an acyclic covalent bond. A Newman projection of a chair conformation of cyclohexane clearly shows that torsional strain is minimized since all groups are staggered. The chair structure of cyclohexane is considered to be the perfect conformation. Stability Of Cyclohexane Type Species Chemistry Stack Exchange.

In each of the boxes below draw in methyl Me groups in the appropriate positions. O H H H H H H H H H H O. For aldoses having high stability in one chair conformation the rates of oxidation of. The preferred conformation of the tetrahydropyran ring is the chair conformation. As one might expect the tetrahydropyran ring adopts a chair conformation and is largely similar to cyclohexane. And using the ratio 955 it is calculated that this corresponds to 728 kJmol. Draw The Chair Conformations Of Each Of The Following Molecules Indicating Which One Should Be More Stable And Briefly Justify Your Reasoning Study Com.

Cyclohexane and the Chair Structure. In glucose all the hydroxy groups are in the equatorial position. We can now move arond the exterior oxygen molecules to find in which postition equatorial or axial the glucose reaches its lowest energy level. What is this position and what is the energy level kJmol. Thus the energy barrier for ring flipping is about 10 kcalmol in each case. Draw the compound below in an all chair conformation with an intramo ecular hydrogen bond AS A DOTTED INE between the a cohol proton 10 and the oxygen of the ring 5 points Flat-Ring Chairs clearly showing intramolecular H-Bond between 10 and 1. Cyclohexane Ring An Overview Sciencedirect Topics.
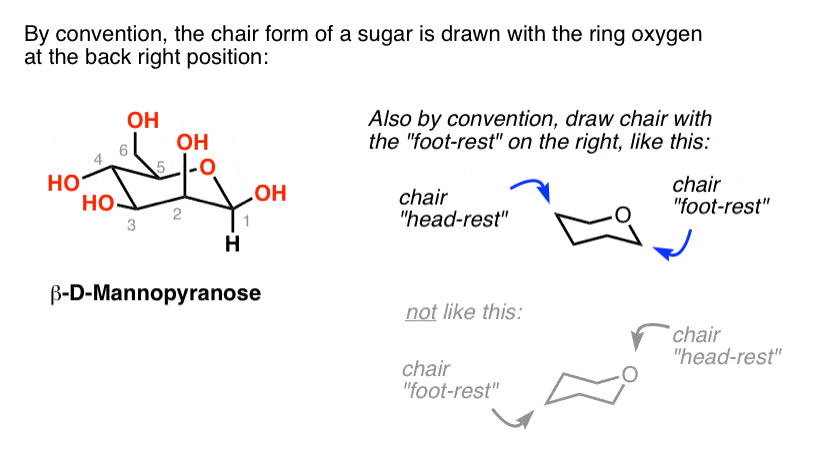
Chair conformations are easily drawn by recognizing the differences between the sugar in question and glucose. The symmetry is D3d. That forms between the oxygen atom on C-5 and the hemiacetal carbon atom C-1 is usually shown by using a box in the Fischer projection. The larger the group the higher the energy difference. Ive found that some of the best depictions of six-member-ring conformations come from pyranose sugars. Draw the Newman projections of the lowest energy conformation looking through the C3-C4 bond for the following. The Haworth Projection Master Organic Chemistry.
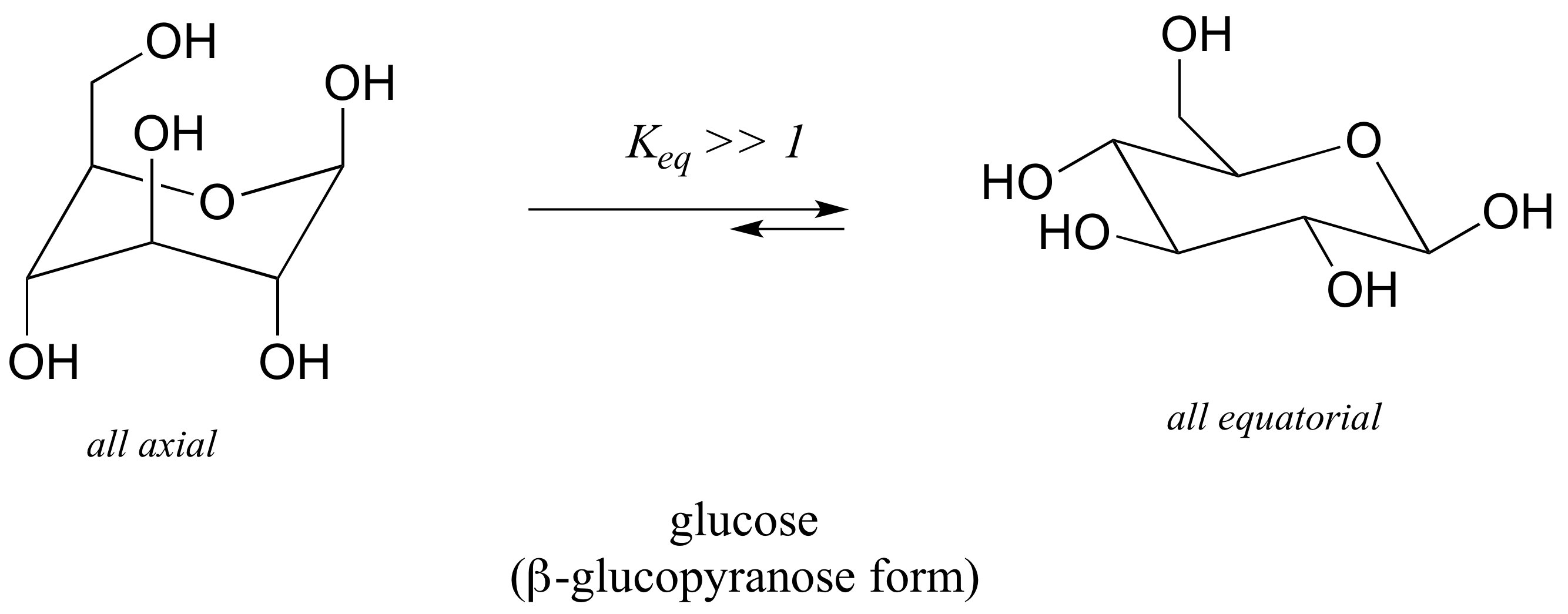
This first conformation is called the chair conformation. Download to read the full article text Literature cited 1. Explain with a drawing why this is the case 2 marks. In both chair conformations is so low that they probably exist in a variety of conformations the rates of ox idation of the anomers show little difference and no particular correlation with the angular position of the Cl-hydroxyl group. For benzylidenecyclohexanone oxide a chair conformation with an axial oxygen atom of the epoxy group is preferential. Draw the compound below in an all chair conformation with an intramo ecular hydrogen bond AS A DOTTED INE between the a cohol proton 10 and the oxygen of the ring 5 points Flat-Ring Chairs clearly showing intramolecular H-Bond between 10 and 1. 3 3 Conformations Of Cyclic Organic Molecules Chemistry Libretexts.










