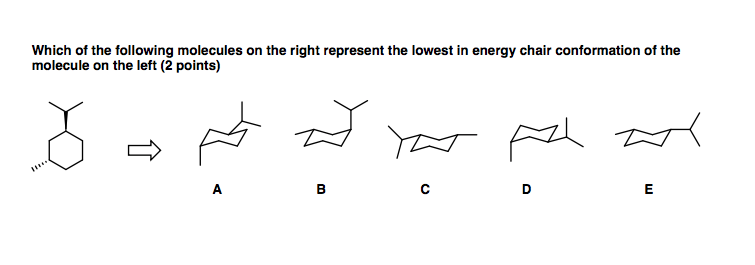
chair conformation ratio The steps involved in drawing the chair conformation of cyclohexane. Construct a model of trans14-dibromocyclohexane in its lower energy conformation.
Chair Conformation Ratio, A chair conformation with an axial substituent is less stable compared to a chair conformation with an equatorial substituent. These two chair conformations are the most common and comfortable of all the conformational possibilities available to a cyclohexane ring. How can I draw for cis -1-ethyl-3-methylcyclohexane the.
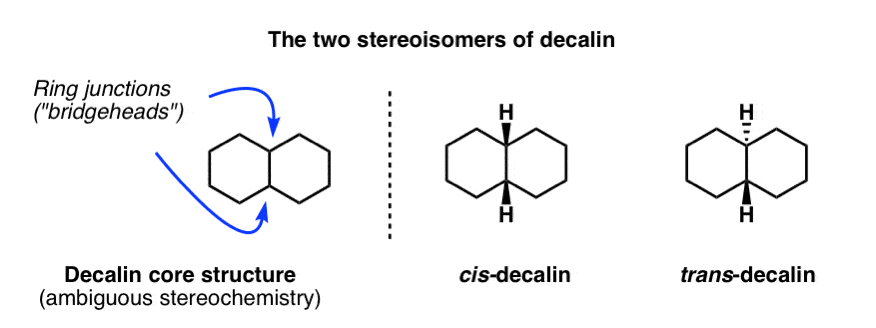 Fused Rings Cis And Trans Decalin Master Organic Chemistry From masterorganicchemistry.com
Fused Rings Cis And Trans Decalin Master Organic Chemistry From masterorganicchemistry.com
The reason for lower stability is in steric interactions between the axial substituent and the axial hydrogens on the same side of the ring Fig. The ratio of two chair conformations in the following mixture is 95. Invert the chair into the alternate chair. Almost all of your work with cyclohexanes will involve chair conformations. Cis Trans Chair Conformation.
Which is more stable cis- or trans- isomers.
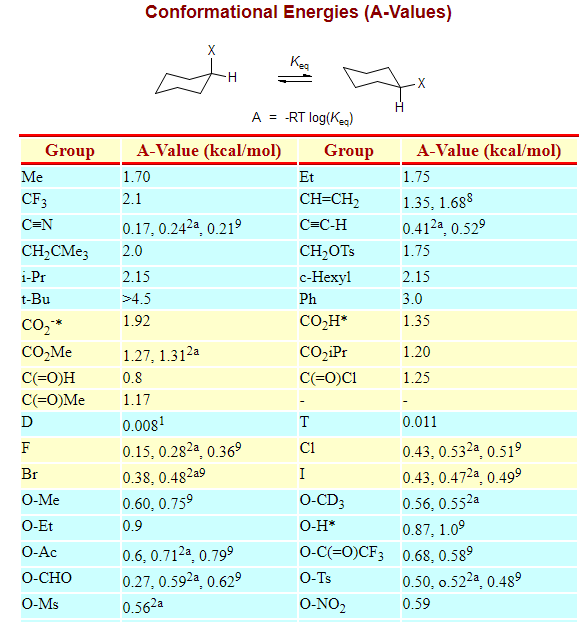
Notice that half of the hydrogen atoms have a different chemical environment. One axial group increases the potential energy by an amount listed in the table for that substituent. In the first conformer we have two chlorines in axial positions so the total steric strain is. The chair is by far the most stable and only the skew conformation has an energy minimum in a similar range but this is still some 20 kJ higher than the chair. Cis Trans Chair Conformation.
Another Article :
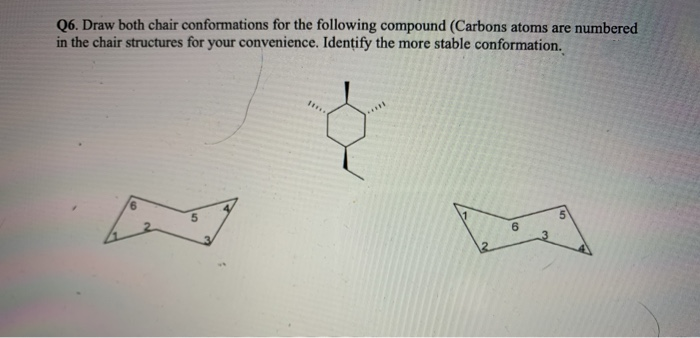
If two axial substituents are on the same side cis add an additional 2 kcalmole to the sum of. The alpha-anomer 4C1 chair conformations were found to be approximately 1 kcalmol lower in electronic energy than the beta-anomers. In cyclohexane derivatives the two chair conformers interconvert with rapidly at room temperature with cyclohexane itself undergoing the ring-flip at a rates of approximately 10 5 ring-flipssec with an overall energy barrier of 10 kcalmol 42 kJmol which precludes their separation at ambient temperatures. This difference corresponds to a equatorialaxial conformer ratio of 191 at 25 C. In a chair there are three carbons that are like mountain peaks red balls and three that are notches blue balls. The most stable conformation of methylcyclohexane is the chair conformation in which the methyl group is equatorial. Answered Q6 Draw Both Chair Conformations For Bartleby.

5 in favor of the one where the methyl group is equatorial. How can I draw for cis -1-ethyl-3-methylcyclohexane the. One axial group increases the potential energy by an amount listed in the table for that substituent. 5 in favor of the one where the methyl group is equatorial. The hydroxymethyl gt conformation was of lowest electronic energy for both the alpha- and beta-anomers. In cyclohexane derivatives the two chair conformers interconvert with rapidly at room temperature with cyclohexane itself undergoing the ring-flip at a rates of approximately 10 5 ring-flipssec with an overall energy barrier of 10 kcalmol 42 kJmol which precludes their separation at ambient temperatures. Fused Rings Cis And Trans Decalin Master Organic Chemistry.

Given your value in a calculate the percent of the chair indicated as B presented in an equilibrium mixture of the conformers at 25ºC. How can I draw for cis -1-ethyl-3-methylcyclohexane the. Draw the second chair conformation ring-flip-check this post if not sure. Then you should familiarize yourself with the geometry of chair conformation. A particularly important case comes up with. Next add a downward-pointing V tip to one end this is the tail of the chair. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.

Cis Trans Chair Conformation. Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign. And now the stabilities. The ratio anti. Therefore the chair conformation is more stable than boat conformation at room temperature. It is shifted to the more stable chair conformation. Blog 05 Calculate Percentage Of Chair Conformation In Cyclohexane.
What is the energy difference between axial and equatorial conformers of methylcyclohexane. If you have any question ask me any time on our homepage. The most important conformations for a cyclohexane molecule are chair half-chair boat and twist-boat. Draw the second chair conformation ring-flip-check this post if not sure. Notice that half of the hydrogen atoms have a different chemical environment. Lets take for example cyclohexane. Blog 05 Calculate Percentage Of Chair Conformation In Cyclohexane.
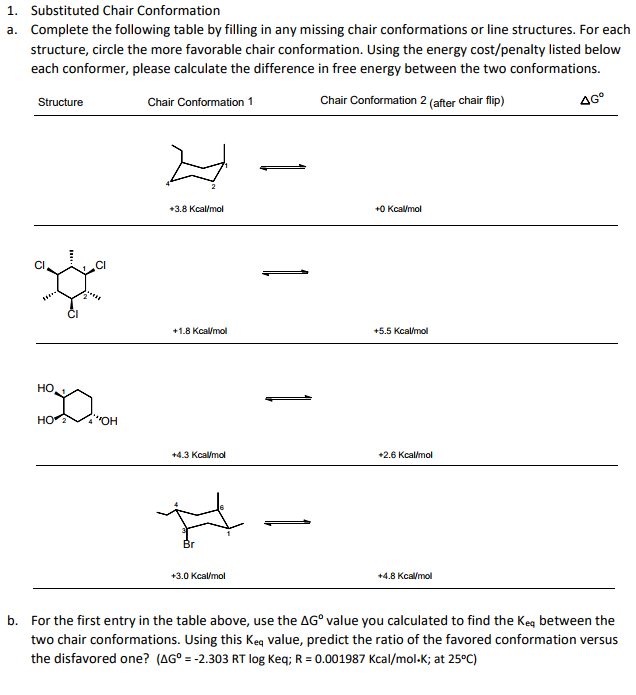
In this compound conformation having Br equatorial is present in 668 and having Br at axial position is present in 332 at 20C. Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign. Invert the chair into the alternate chair. Lets take for example cyclohexane. The steps involved in drawing the chair conformation of cyclohexane. The following guidelines can be used to determine the percent distribution of two chair conformers using the Gibbs free energy equation and some simple algebra. Substituted Chair Conformation Complete The Following Chegg Com.

What is the orientation of the two C-Br bonds ie axial or equatiorial. What is the orientation of the two C-Br bonds now. The peaks have axial up and notches have axial. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. Almost all of your work with cyclohexanes will involve chair conformations. If you have any question ask me any time on our homepage. 7 16 E2 Regiochemistry And Cyclohexane Conformations Chemistry Libretexts.

Therefore the chair conformation is more stable than boat conformation at room temperature. Next add a downward-pointing V tip to one end this is the tail of the chair. Therefore the chair conformation is more stable than boat conformation at room temperature. One axial group increases the potential energy by an amount listed in the table for that substituent. For each chair conformer add the energy of all the groups on axial position. What is the reason for this. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.
Notice that half of the hydrogen atoms have a different chemical environment. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. The chair conformation is the most stable conformation of cyclohexane Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring The bond angles in this conformation are 1109. Other principal conformations of pyranoses are halfchair H boat B and skew S conformation which are named as indicated. The peaks have axial up and notches have axial. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. Blog 05 Calculate Percentage Of Chair Conformation In Cyclohexane.
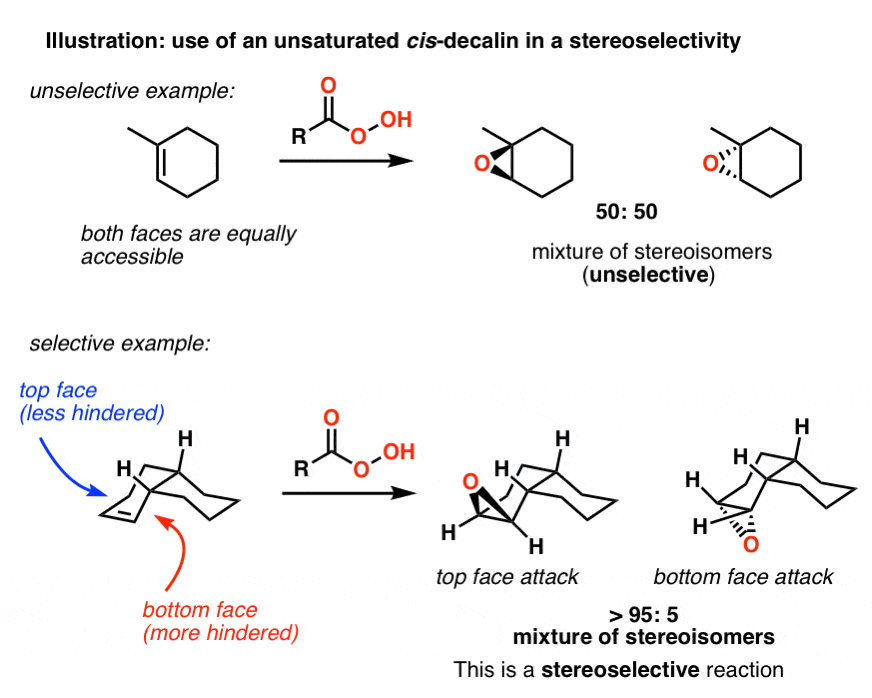
The chair is by far the most stable and only the skew conformation has an energy minimum in a similar range but this is still some 20 kJ higher than the chair. There are two chair conformations for each cyclohexane example above. Number the ring and draw any chair conformation of the compound. These structures when arranged according to their stabilities are chairtwist boatboathalf-chair. Other principal conformations of pyranoses are halfchair H boat B and skew S conformation which are named as indicated. How can I draw for cis -1-ethyl-3-methylcyclohexane the. Fused Rings Cis And Trans Decalin Master Organic Chemistry.

Number the ring and draw any chair conformation of the compound. The most stable conformation of methylcyclohexane is the chair conformation in which the methyl group is equatorial. The glucose alphabeta anomer ratio calculated from the relative free energies is 6337. The free energy ΔG of the equilibrium process going from one conformer to the other ie from A to B as shown in step 4 or 5 can be calculated from the equation below where the. It is shifted to the more stable chair conformation. The reason for lower stability is in steric interactions between the axial substituent and the axial hydrogens on the same side of the ring Fig. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.

The peaks have axial up and notches have axial. Then you should familiarize yourself with the geometry of chair conformation. The steps involved in drawing the chair conformation of cyclohexane. A chair conformation with an axial substituent is less stable compared to a chair conformation with an equatorial substituent. The free energy ΔG of the equilibrium process going from one conformer to the other ie from A to B as shown in step 4 or 5 can be calculated from the equation below where the. In this compound conformation having Br equatorial is present in 668 and having Br at axial position is present in 332 at 20C. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.
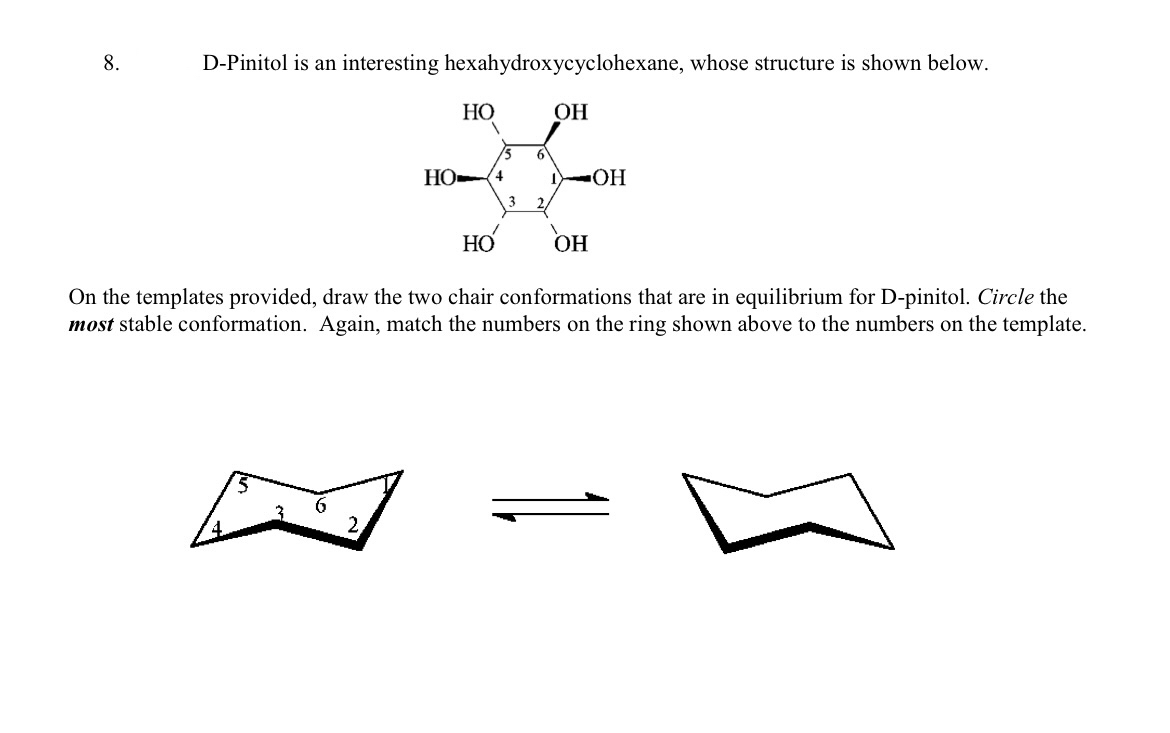
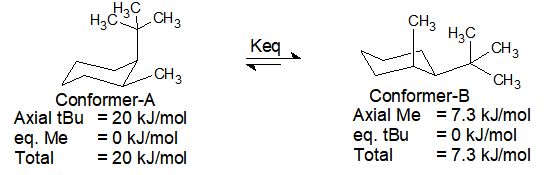
Lets take for example cyclohexane. The glucose alphabeta anomer ratio calculated from the relative free energies is 6337. Notice that half of the hydrogen atoms have a different chemical environment. The alternative chair conformation in which the methyl group is axial is 73 kJmol higher in energy. The chair is by far the most stable and only the skew conformation has an energy minimum in a similar range but this is still some 20 kJ higher than the chair. One axial group increases the potential energy by an amount listed in the table for that substituent. Answered 8 D Pinitol Is An Interesting Bartleby.
Therefore the chair conformation is more stable than boat conformation at room temperature. Construct a model of trans14-dibromocyclohexane in its lower energy conformation. One axial group increases the potential energy by an amount listed in the table for that substituent. Then you should familiarize yourself with the geometry of chair conformation. There are two chair conformations for each cyclohexane example above. Lets take for example cyclohexane. Chair Conformations Homework Assignment 5 Note Chegg Com.
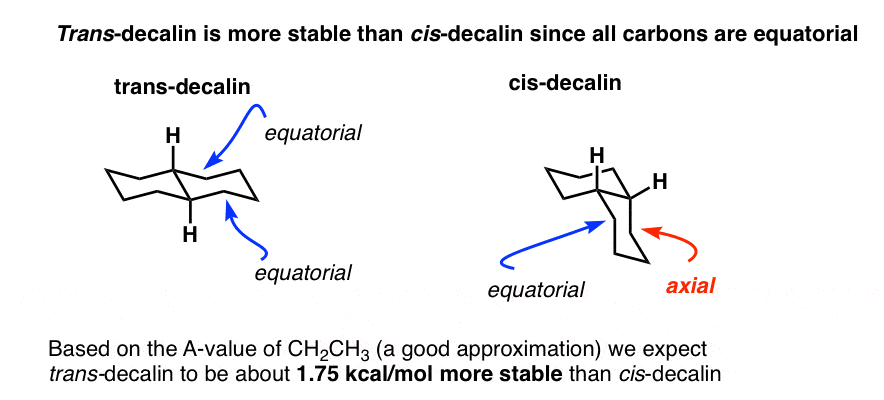
The steps involved in drawing the chair conformation of cyclohexane. There are two chair conformations for each cyclohexane example above. Identify any 13-diaxial interactions present in both conformers. Then you should familiarize yourself with the geometry of chair conformation. What is the energy difference between axial and equatorial conformers of methylcyclohexane. What is the reason for this. Fused Rings Cis And Trans Decalin Master Organic Chemistry.