chair conformation point group Dipotassium Pentasulfide K 2 S 5 Lithium nitride Li 3 N Na 172 In 192 Pt 2. OH GROUP POINTS DOWN- AXIAL POSITION α OH GROUP POINTS UP EQUATORIAL POSITION- β.
Chair Conformation Point Group, How to tell a isomer in chair conformation is cis or trans. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Additionally either bond-line or Newman formulas reveal that the two hydrogen atoms at each CH2 are not the same.
 4 3 Conformations Of Cyclic Organic Molecules Chemistry Libretexts From chem.libretexts.org
4 3 Conformations Of Cyclic Organic Molecules Chemistry Libretexts From chem.libretexts.org
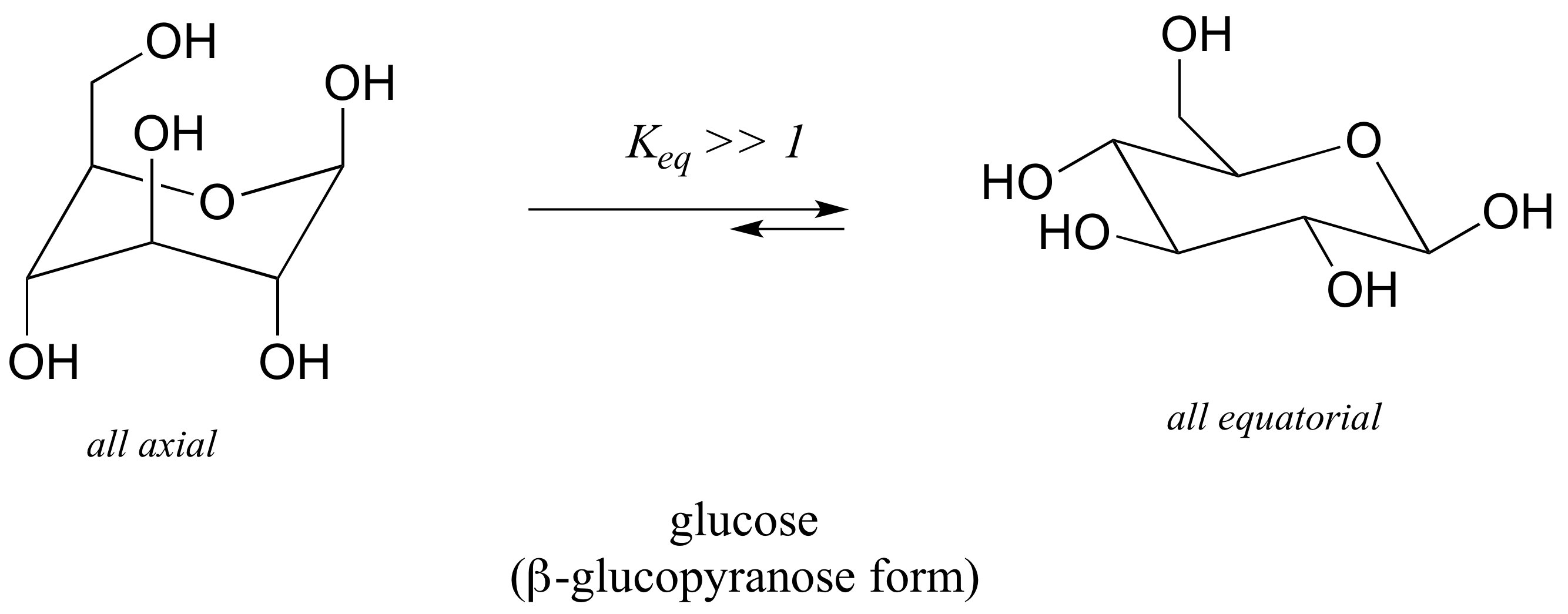
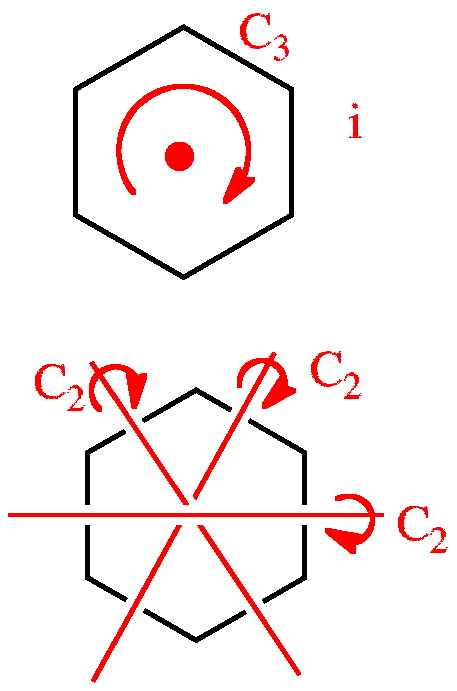
Of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the aircraft conformation from the D3d symmetry group is called a form of chair. The collection of symmetry elements present in a molecule forms a group typically called a point group. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. Each Equatorial group becomes Axial and each Axial group becomes Equatorial. The most stable conformation is the β chair conformation since it reduces steric hindrance.
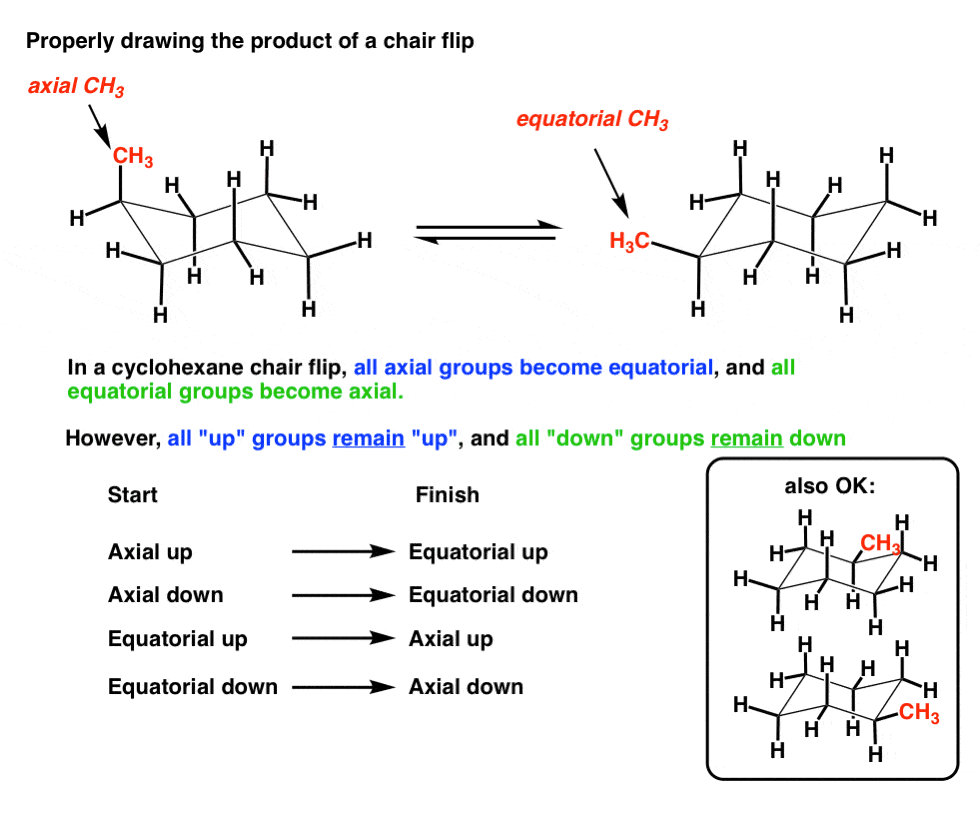
All the groups pointing up should still be pointing up regardless of being axial or equatorial.
The boat and chair conformations are indeed symmetric and achiral. Chair half-chair twist-boat half-chair chair. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. Note that in chair cyclohexane there are two different types of C-H bonds and thus two chemically different types of hydrogens. The interconversion between the two chair conformations involves the following sequence.
Another Article :
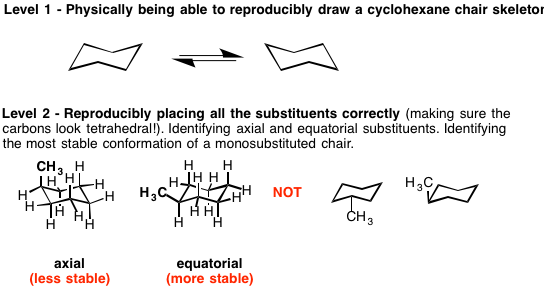
For example the staggered and ecliptic conformations of ethane or the chair and boat conformations of. Additionally either bond-line or Newman formulas reveal that the two hydrogen atoms at each CH2 are not the same. There are 6 of these 3 upward and 3 downward bonds and. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. In the chair conformation to determine whether either or is α or β LOOK AT THE OH GROUP. A general way to recognize is to check that whether a group attached by the bond is above the ring point up or below the ring point down. Levels Of Mastery Master Organic Chemistry.

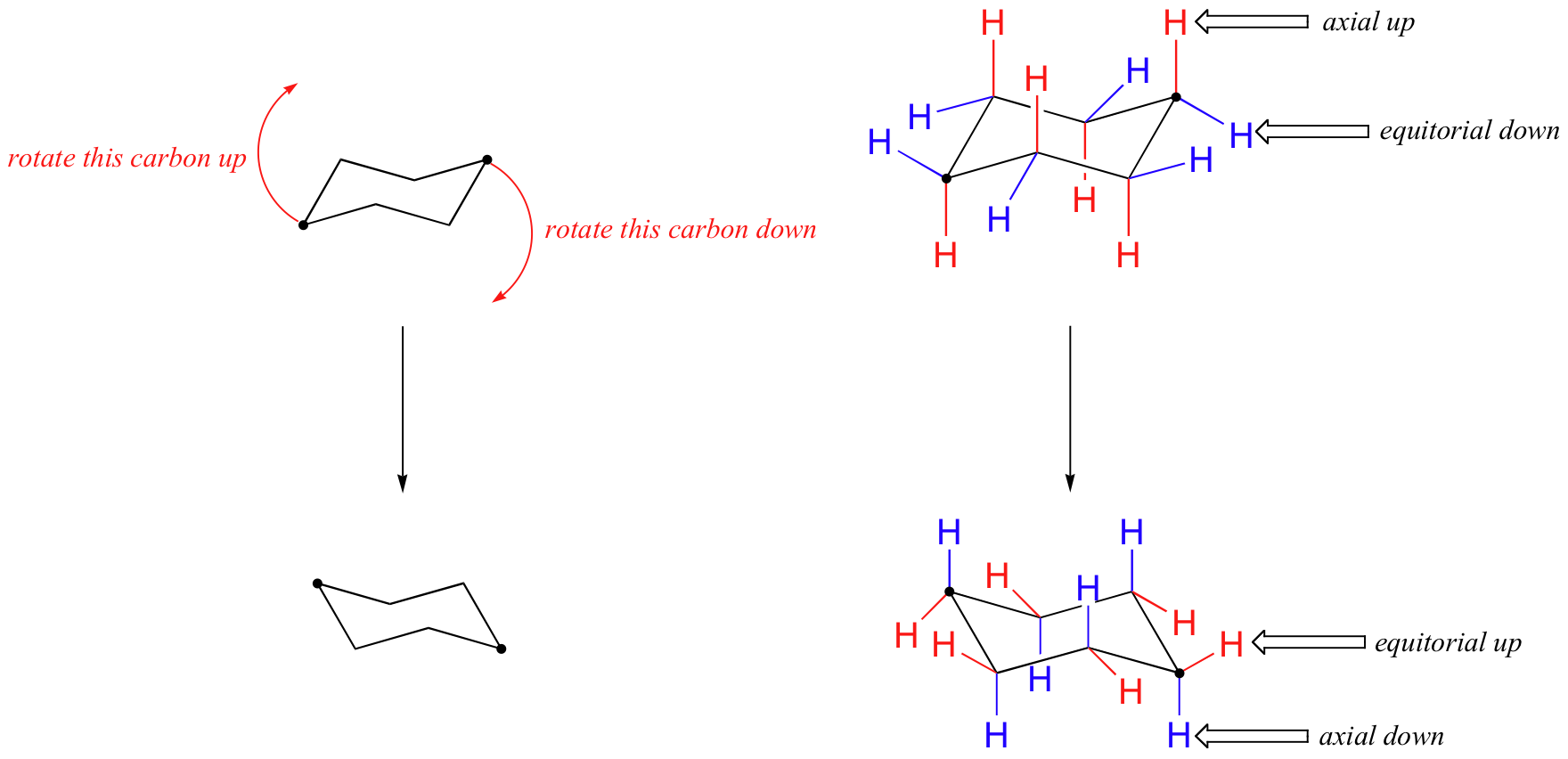
Of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the aircraft conformation from the D3d symmetry group is called a form of chair. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top point. OH GROUP POINTS DOWN- AXIAL POSITION α OH GROUP POINTS UP EQUATORIAL POSITION- β. The interconversion between the two chair conformations involves the following sequence. The chair structure of cyclohexane is considered to be the perfect conformation. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. Cyclohexane Chairs Equatorial Groups Can Be Up Or Down Too Organic Chemistry Study Organic Chemistry Teaching Chemistry.
Which one is more stable out of these two. The C-H bonds which point vertically upward or downward are called axial. The idea that the chair conformation is the most stable structure for cyclohexane was first proposed as early as 1890 by Hermann Sachse but only gained widespread acceptance much later. The chair conformation is the most stable conformation of cyclohexane. Point group is a collection of symmetry elements present in a molecule that obeys the mathematical rules for the formation of a group. Calcium Carbonate CaCO 3 Polymorphs. A The Chair Conformation Of Cyclohexane B Representation Of The Download Scientific Diagram.

The same term applies to similar conformations of six-member saturated ring analogous structures. The twist-boat or skew conformation has point group D 2 and is indeed chiral. A step-by step procedure for drawing the chair form in. The chair conformation in which the methyl group is equatorial is the most stable while the alternative chair conformation in which the methyl group is axial is 73 kJmol more energetic. The same term applies to similar conformations of six-member saturated ring analogous structures. The more stable chair conformation of trans-12-dimethylcyclohexane has the two methyl groups in the equatorial position. 4 6 Axial And Equatorial Bonds In Cyclohexane Chemistry Libretexts.

Contrary to the case of methylcyclohexane which has no interactions in the chair conformation having an equatorial methyl group the diequatorial conformer of trans-12-dimethylcyclohexane has a gauche butane interaction red carbon atoms between the two methyl groups. The point group assignment depends on how the pairs of spokes attached to both the front and back of the hub connect with the rim. There are 6 of these 3 upward and 3 downward bonds and. The twist-boat or skew conformation has point group D 2 and is indeed chiral. What is the most stable chair conformation. The chair conformation is the most stable conformation of cyclohexane. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
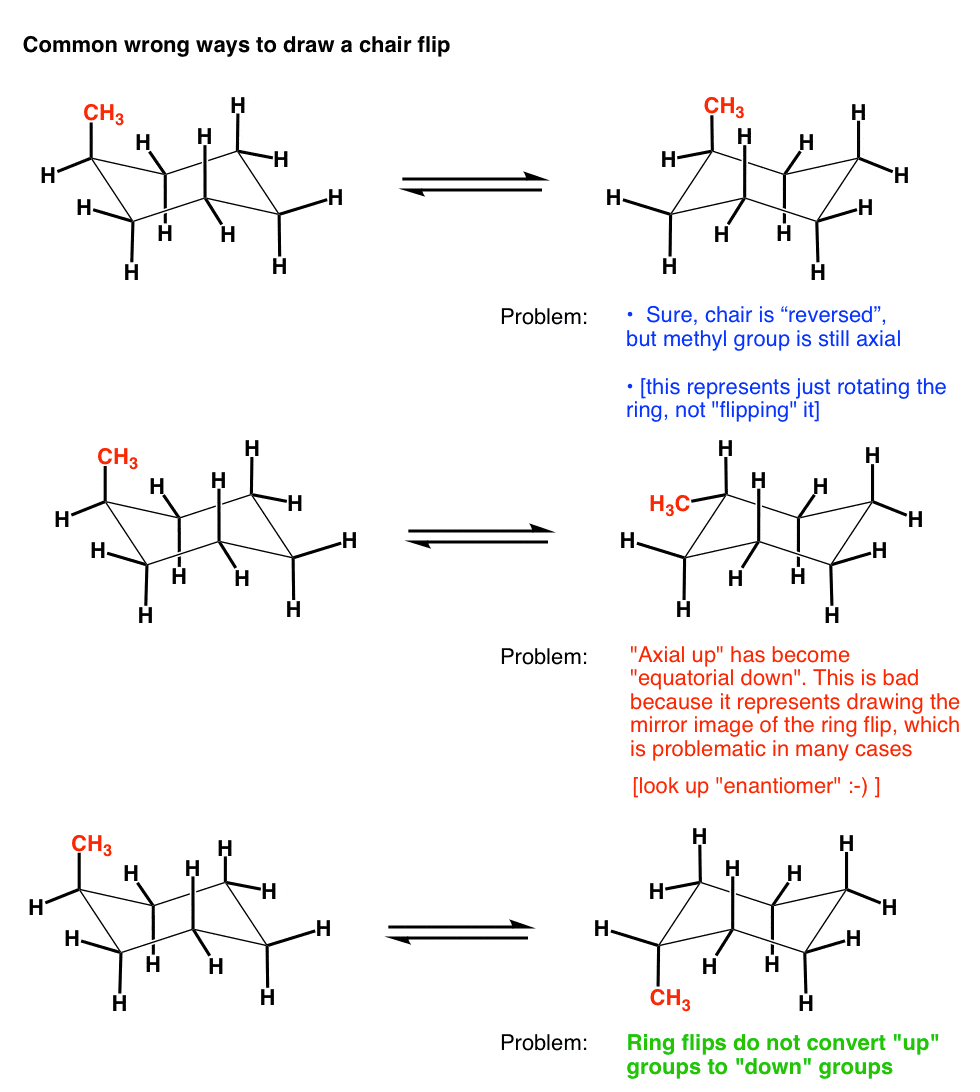
OH GROUP POINTS DOWN- AXIAL POSITION α OH GROUP POINTS UP EQUATORIAL POSITION- β. Zeolites X. There are 6 of these 3 upward and 3 downward bonds and. If both groups point to the same side the compound is cis isomer. A Newman projection of a chair conformation of cyclohexane clearly shows that torsional strain is minimized since all groups are staggered. Ok Here we go again. The Cyclohexane Chair Flip Master Organic Chemistry.
Calcium Carbide CaC 2. How to tell a isomer in chair conformation is cis or trans. The line of sight is parallel to two bonds in the chair as shown below where axial and equatorial substituents are indicated as a and e respectively. The boat and chair conformations are indeed symmetric and achiral. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. What is the most stable chair conformation. Cyclohexane Chair D3d.
If the pairs alternate with respect to their side of attachment the point group is D8d. A Newman projection of a chair conformation of cyclohexane clearly shows that torsional strain is minimized since all groups are staggered. The collection of symmetry elements present in a molecule forms a group typically called a point group. Look at the two figures below and see that they do contain the identical set of symmetry elements even though their overall shapes are quite different. Orient a model such that you can clearly see the view from this perspective. Scandium Chloride Sc 7 Cl 10. The Cyclohexane Chair Flip Master Organic Chemistry.
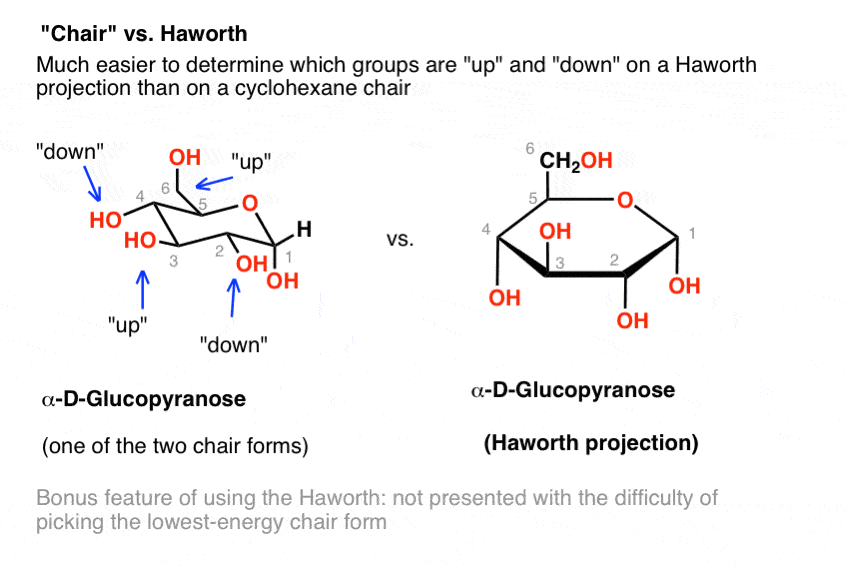
That is about it for drawing the ring-flip of chair conformations. K 4 Ge 4 Cs18-crown-6 2 e Group 2 Elements. Point group is a collection of symmetry elements present in a molecule that obeys the mathematical rules for the formation of a group. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top point. Which one is more stable out of these two. The most stable conformation is the β chair conformation since it reduces steric hindrance. The Haworth Projection Master Organic Chemistry.
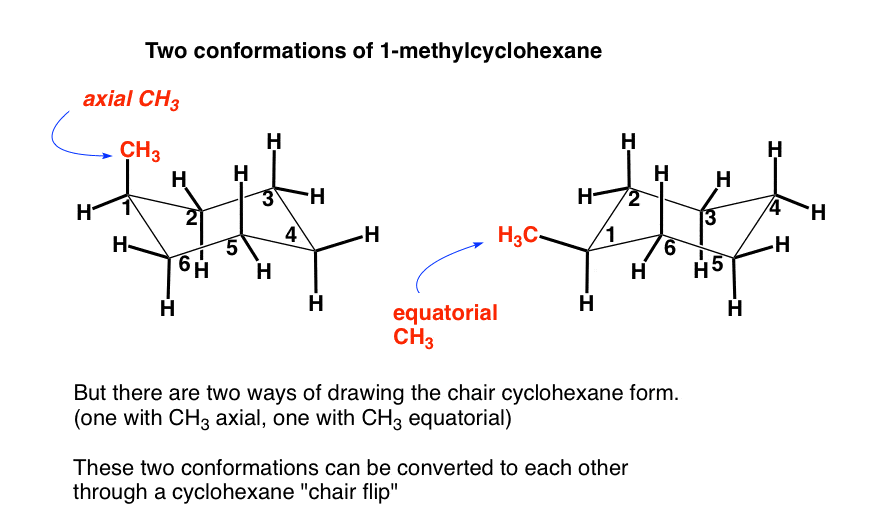
Chair Conformation and Ring Flips - YouTube. There are 6 of these 3 upward and 3 downward bonds and. Every carbon on the chair conformation has. Look at the two figures below and see that they do contain the identical set of symmetry elements even though their overall shapes are quite different. The chair conformation in which the methyl group is equatorial is the most stable while the alternative chair conformation in which the methyl group is axial is 73 kJmol more energetic. If both groups point to the same side the compound is cis isomer. The Cyclohexane Chair Flip Master Organic Chemistry.
Zeolites X. Scandium Chloride Sc 7 Cl 10. Caesium Peroxide Cs 2 O 2. An envelope conformation will have a plane of symmetry and thus be achiral. What is the most stable chair conformation. A general way to recognize is to check that whether a group attached by the bond is above the ring point up or below the ring point down. 4 6 Axial And Equatiorial Bonds In Cyclohexane Chemistry Libretexts.

In the common notation aka Schoenflies notation this is known as the C 2v point group. Scandium Chloride Sc 7 Cl 10. The same term applies to similar conformations of six-member saturated ring analogous structures. Of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the aircraft conformation from the D3d symmetry group is called a form of chair. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. Dipotassium Pentasulfide K 2 S 5 Lithium nitride Li 3 N Na 172 In 192 Pt 2. 1 3 Diaxial Gauche Interactions Chair Methylcycohexane Interactive Chemistry Free Energy.
The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Cyclohexane and the Chair Structure. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. A step-by step procedure for drawing the chair form in. The line of sight is parallel to two bonds in the chair as shown below where axial and equatorial substituents are indicated as a and e respectively. Chair Conformation and Ring Flips - YouTube. 4 6 Axial And Equatorial Bonds In Cyclohexane Chemistry Libretexts.
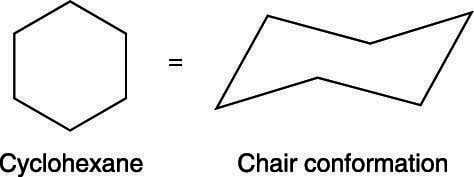
Zeolites X. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Calcium Carbide CaC 2. Another molecule that also belongs to the C 2v point group is cyclohexane in the boat conformation. Calcium Carbonate CaCO 3 Polymorphs. Without knowledge of the exact configuration or conformation the determination of its point group is impossible. How To Draw The Chair Conformation Of Cyclohexane Dummies.
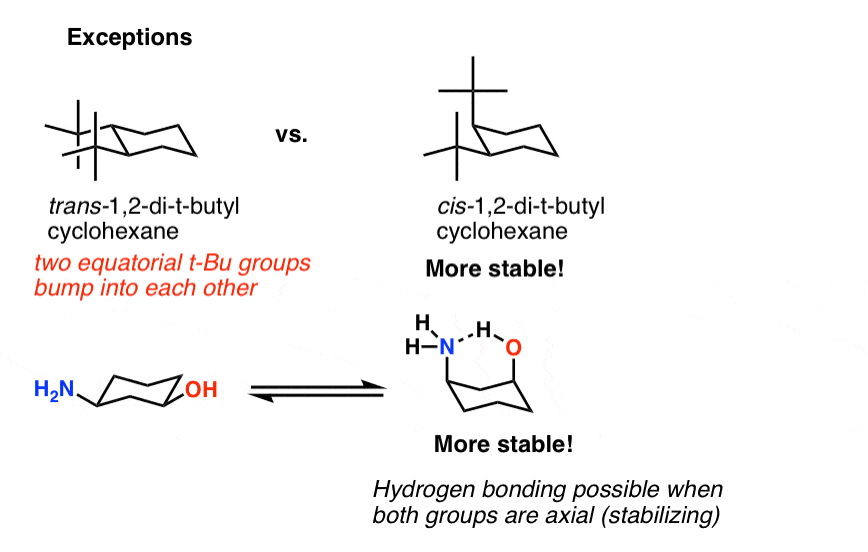
The C-H bonds which point vertically upward or downward are called axial. Every carbon on the chair conformation has. It is important to note that the point groups to which a molecule belongs ultimately depends on its exact molecular geometry and its specific configuration and conformation. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. The chair structure of cyclohexane is considered to be the perfect conformation. The chair conformation in which the methyl group is equatorial is the most stable while the alternative chair conformation in which the methyl group is axial is 73 kJmol more energetic. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.











