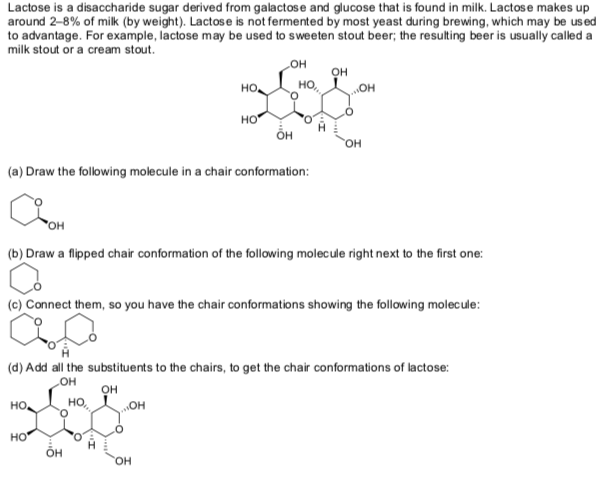
chair conformation of lactose Lactose is a disaccharide that yields D-glucose and D-galactose on hydrolysis. Constituents of the ring that project above or below the plane of the ring are axial and those that project parallel to the plane are equatorial.
Chair Conformation Of Lactose, Celluose molecules within the plant cell walls are organized into biological units of structure known as microfibrils. Below is the structure of lactose the sugar found in dairy products. 43 The C2 Chirality and the Ring FormConformation.
 Role Of Lactose Phosphate In Lactose Containing Dairy Products Semantic Scholar From semanticscholar.org
Role Of Lactose Phosphate In Lactose Containing Dairy Products Semantic Scholar From semanticscholar.org
General considerations3-6 suggest that in f-lactose the chair conformation of the pyranoid moieties is retained but the twist around the COC bridge could well be quite different. Fischer Projections Haworth Structures and Chair Conformers The acyclic structure of a sugar is commonly drawn as a Fischer projection. Celluose molecules within the plant cell walls are organized into biological units of structure known as microfibrils. It is designated as 4-0-β-galactopyranosyl-D-glucopyranose and occurs in both alpha and beta forms. From the time that a C 9 structure with a keto group on C2 and an acetamido group at C5 was generally accepted for sialic acid a pyranose ring form between C2 and C6 was drawn although still in a Fischer projection formula.
Chair conformation for each of the sugars present in this carbohydrate.
In the chair conformation the orientation of the OH group about the anomeric carbon of α-D-glucose is axial and equatorial in β-D-glucose. The equilibrium between both forms is established by mutarotation. People who are lactose intolerant do not produce enough lactase - the enzyme that hydrolyzes the glycosidic bond linking the two monosaccharides - to be able to fully digest dairy products. To absorb its components and use them for energy you digest it with lactase an enzyme produced by your digestive tract. From the time that a C 9 structure with a keto group on C2 and an acetamido group at C5 was generally accepted for sialic acid a pyranose ring form between C2 and C6 was drawn although still in a Fischer projection formula.
Another Article :

The chair conformation cannot deform without changing the bond angles or lengths. Lactase reacts with lactose splitting it into two smaller sugar molecules that you can absorb. Celluose molecules within the plant cell walls are organized into biological units of structure known as microfibrils. You consume it any time you drink milk or eat dairy products. In the chair conformation the orientation of the hydroxyl group about the anomeric carbon of α-D-glucose is axial and equatorial in β-D-glucose. It is an aldohexose and a C-4 epimer of glucose. Anomeric Forms Of The Carbohydrates As A And B Pyranose In The 4 C 1 Download Scientific Diagram.
Conformations Lactose chair -D-Galactose à à à à à à à ã -d-glucose -1 4-glycoside sucrose -d- glucose à à à à à à à à ã ã ã ã -d-fructose -1 screenings haworth 2-glycoside of lactose and sucrose. Notes Lactose is made from a d-glucose molecule covalently bonded to a d-galactose molecule via a b-14-glycosidic linkage. 43 The C2 Chirality and the Ring FormConformation. Chair conformation for each of the sugars present in this carbohydrate. Galactose is a simple sugar formed when lactose a carbohydrate in milk is hydrolyzed. Both sugars 1 and 2 are D-glucose as shown below. Solved Lactose Is A Disaccharide Sugar Derived From Chegg Com.

The chair conformation cannot deform without changing the bond angles or lengths. Lactose usually crystallizes in the form of its alpha-lactose monohydrate. Celluose molecules within the plant cell walls are organized into biological units of structure known as microfibrils. It is an aldohexose and a C-4 epimer of glucose. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. CHO H OH HO H H OH H OH CH 2OH E. Draw The Chair Conformations Of Each Of The Following Molecules Indicating Which One Should Be More Stable And Briefly Justify Your Reasoning Study Com.
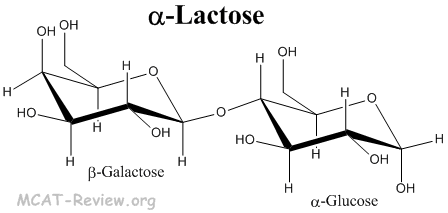
Problem 4 Easy Difficulty. Galactose is a simple sugar formed when lactose a carbohydrate in milk is hydrolyzed. People who are lactose intolerant do not produce enough lactase - the enzyme that hydrolyzes the glycosidic bond linking the two monosaccharides - to be able to fully digest dairy products. Crystalline lactose is the α-anomer and exhibits mutarotation when dissolved. Glucose can assume both but it is more stable in chair formation since carbon 2345 and 6 lie on the same plane while C1 lies above the plane and C4 below. Lactose is a disaccharide that yields D-glucose and D-galactose on hydrolysis. Carbohydrates Biological Molecules Mcat Review.
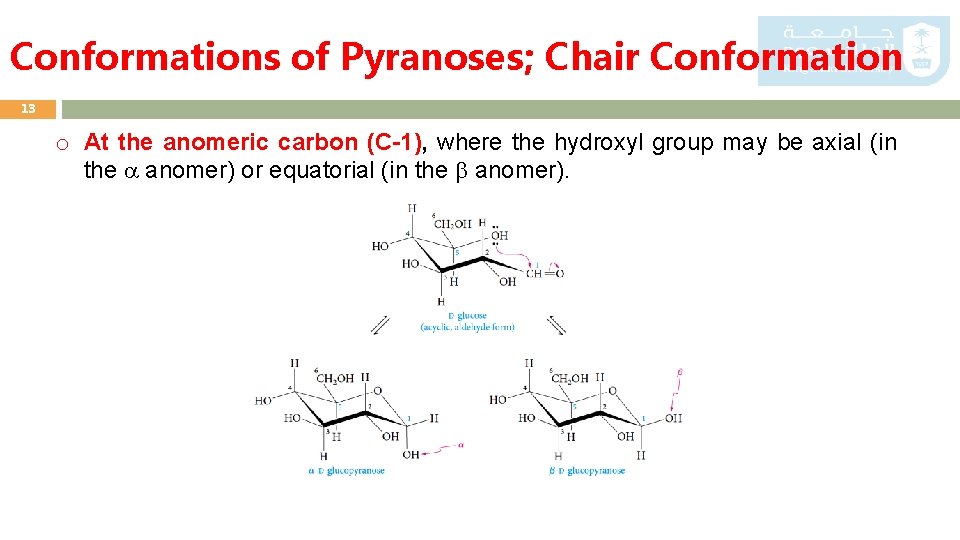
The chair conformation cannot deform without changing the bond angles or lengths. People who are lactose intolerant do not produce enough lactase - the enzyme that hydrolyzes the glycosidic bond linking the two monosaccharides - to be able to fully digest dairy products. O CH 2OH HO HO HO OH D. According to the type of sugar its conformation can either assume a boat or chair formation. Ball-and-stick model and chair conformation of the disaccharide lactose. CHO H OH HO H H OH H OH CH 2OH E. Fundamentals Of Organic Chemistry Chem 109 For Students.

The predominant carbohydrates encountered in the body are structurally related to the aldotriose glyceraldehyde and to the ketotriose dihydroxyacetone. A galactose molecule linked with a glucose molecule forms a lactose molecule. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. Ball-and-stick model and chair conformation of the disaccharide lactose. The chair form is the more stable of the two. It is designated as 4-0-β-galactopyranosyl-D-glucopyranose and occurs in both alpha and beta forms. Figure Un Title D Glucose And D Fructose Caption Ppt Download.

Both sugar moieties adopt a strainless chair conformation. Lactose is a disaccharide that yields D-glucose and D-galactose on hydrolysis. Below is the structure of lactose the sugar found in dairy products. Crystalline lactose is the α-anomer and exhibits mutarotation when dissolved. Roland Schauer Johannis P. Both sugars 1 and 2 are D-glucose as shown below. Galactose Wikiwand.

If the number of heavy axial groups becomes smaller when the conformation is changed to 1C4 all equatorial groups in 4C1 become axial and vice versa then it is likely that the conformation is 1C4. Kamerling in Advances in Carbohydrate Chemistry and Biochemistry 2018. Chair conformation for each of the sugars present in this carbohydrate. Lactose O-β-D-galactopyranosyl-1 - 4-β -D-glucopyranose lactose O OH OH HO HO O O OH HO HO HO Found in milk lactose provides energy to nursing infant Hydrolyzed by laccase. Both sugars 1 and 2 are D-glucose as shown via the chair conformation below. These structures make it easy to show the configuration at each stereogenic center in the molecule without using wedges and dashes. Drawings Elizabeth Canaday.

Below is the structure of lactose the sugar found in dairy products. A galactose molecule linked with a glucose molecule forms a lactose molecule. According to the type of sugar its conformation can either assume a boat or chair formation. The equilibrium between both forms is established by mutarotation. Celluose molecules within the plant cell walls are organized into biological units of structure known as microfibrils. It is designated as 4-0-β-galactopyranosyl-D-glucopyranose and occurs in both alpha and beta forms. Ppt Chair Conformations Powerpoint Presentation Free Download Id 5949148.

Lactose usually crystallizes in the form of its alpha-lactose monohydrate. Celluose molecules within the plant cell walls are organized into biological units of structure known as microfibrils. The predominant carbohydrates encountered in the body are structurally related to the aldotriose glyceraldehyde and to the ketotriose dihydroxyacetone. The chair conformation cannot deform without changing the bond angles or lengths. Lactose is milk sugar. In the chair conformation the orientation of the hydroxyl group about the anomeric carbon of α-D-glucose is axial and equatorial in β-D-glucose. File Chair Conformations Png Wikimedia Commons.
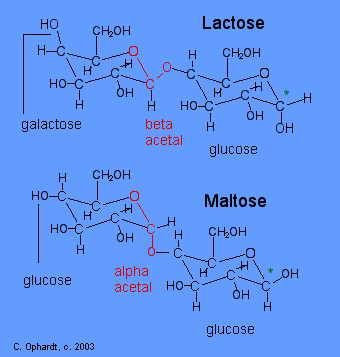
Glucose can assume both but it is more stable in chair formation since carbon 2345 and 6 lie on the same plane while C1 lies above the plane and C4 below. To absorb its components and use them for energy you digest it with lactase an enzyme produced by your digestive tract. Equimolar mixture of glucose and galactose is obtained by hydrolysis of β 1 - 4 glycosidic bonds. If the number of heavy axial groups becomes smaller when the conformation is changed to 1 C 4 all equatorial groups in 4 C 1 become axial and vice versa then it is likely that the conformation is 1 C 4. In aqueous solution lactose consists of 615 of beta-pyranose and 385 of alpha-pyranose. Crystalline lactose is the α-anomer and exhibits mutarotation when dissolved. 23 2 Carbohydrates Chemistry Libretexts.

In the chair conformation the orientation of the OH group about the anomeric carbon of α-D-glucose is axial and equatorial in β-D-glucose. Problem 4 Easy Difficulty. It is designated as 4-0-β-galactopyranosyl-D-glucopyranose and occurs in both alpha and beta forms. People who are lactose intolerant do not produce enough lactase - the enzyme that hydrolyzes the glycosidic bond linking the two monosaccharides - to be able to fully digest dairy products. Lactase reacts with lactose splitting it into two smaller sugar molecules that you can absorb. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. Lactose Intolerance Biochemknowledge.
It is designated as 4-0-β-galactopyranosyl-D-glucopyranose and occurs in both alpha and beta forms. Ball-and-stick model and chair conformation of the disaccharide lactose. Fischer Projections Haworth Structures and Chair Conformers The acyclic structure of a sugar is commonly drawn as a Fischer projection. Chair Conformations For pyranoses the six-membered ring is more accurately represented as a chair conformation O CH2OH HO HO OH OHb C HOH HO CH2OH OHHO O HO OH a HO HO CH2OH O a-D-Glucose b-D-Glucose a-D-Glucopyranose b-D-Glucopyranose D-Glucose anomeric carbon. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. O CH 2OH HO HO HO OH D. Structures Of The Most Common Mono And Disaccharides Carbohydrates Fischer Projections D Glucose D Fructose D Galactose D Ribose 2 Deoxy D Ribose Haworth Projections D Glucose D Glucose D Fructose D Fructose D Galactose D Galactose.

The chair conformation cannot deform without changing the bond angles or lengths. Fischer Projections Haworth Structures and Chair Conformers The acyclic structure of a sugar is commonly drawn as a Fischer projection. General considerations3-6 suggest that in f-lactose the chair conformation of the pyranoid moieties is retained but the twist around the COC bridge could well be quite different. 43 The C2 Chirality and the Ring FormConformation. Galactan is a polymeric form of galactose found in hemicellulose and forming the core of the galactans a class of natural polymeric. Problem 4 Easy Difficulty. Carbohydrates Haworth Fischer Projections With Chair Conformations Youtube.

The equilibrium between both forms is established by mutarotation. The chair form more stable and the boat form. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. Constituents of the ring that project above or below the plane of the ring are axial and those that project parallel to the plane are equatorial. Lactase reacts with lactose splitting it into two smaller sugar molecules that you can absorb. Problem 4 Easy Difficulty. Disaccharides Sucrose Maltose Lactose Carbohydrates Youtube.