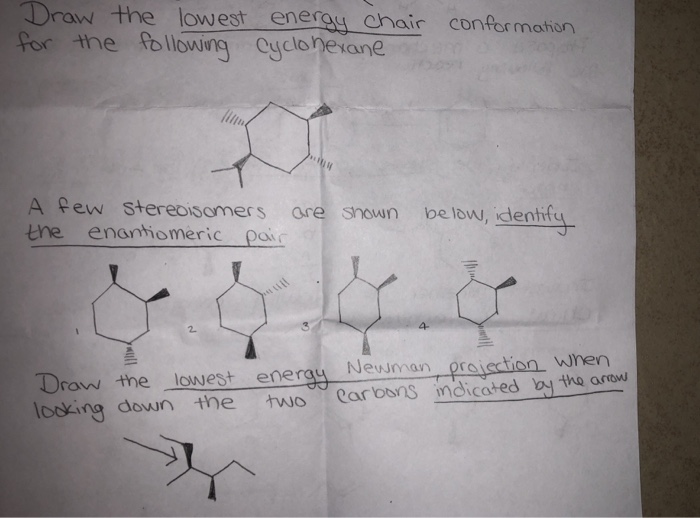
chair conformation lowest energy On careful examination of a chair conformation of cyclohexane we find that the twelve hydrogens are not structurally equivalent. In this conformation the isopropyl group prefers to be attached at the.
Chair Conformation Lowest Energy, Another conformation which is important in any conformational analysis is the transition state or maximum energy conformation on the rotational pathFor cyclohexane this is the so-called half-chair conformation in which now 5 carbons are co-planar and only one is puckered out of the plane. The other conformations are known as the twist and boat forms. And now the stabilities.
 Cyclohexane Chair Conformation Stability Which One Is Lower Energy From masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy From masterorganicchemistry.com
The boat conformation suffers from torsional strain making it less stable higher in energy than the chair. There is no substitute for the use of models. The lowest energy conformation it attains with its tetrahedral carbon atoms simulates a chair. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. The structure above is the most stable structure of trans-1-ethyl-2-isopropylcyclohexane hence it has the lowest energy.
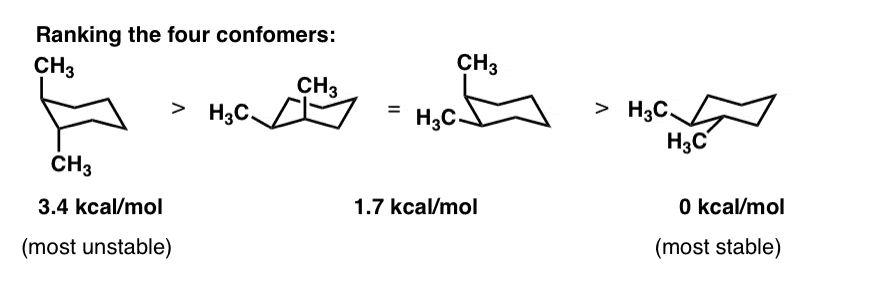
A The two chair conformations are equal in energy.
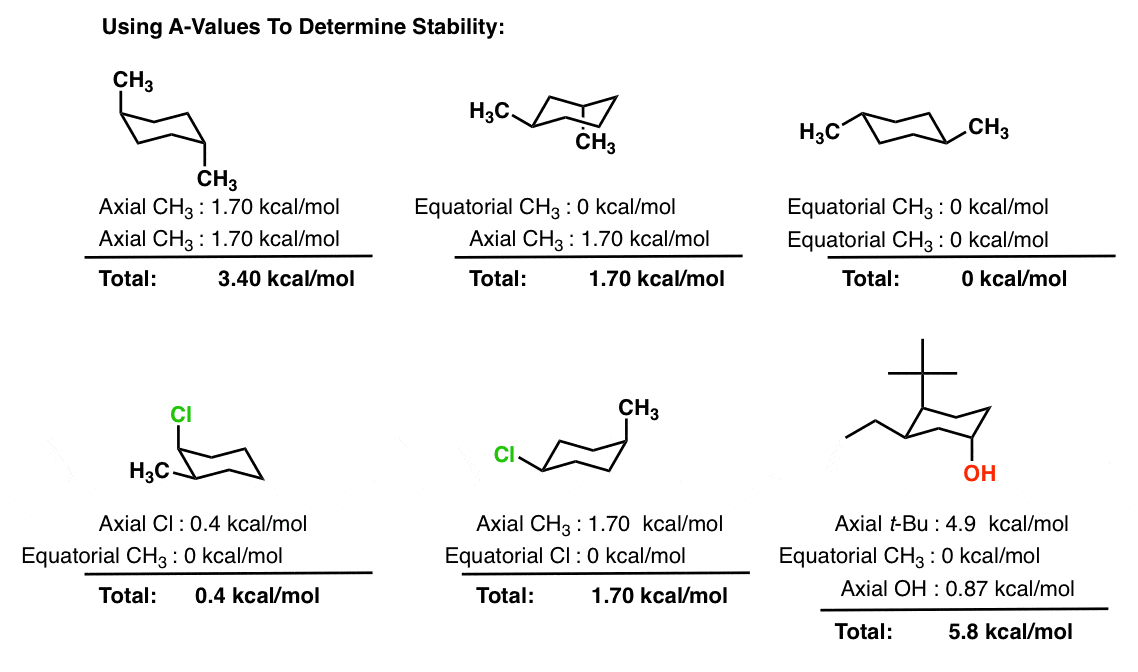
If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. Who are the experts. For each chair conformer add the energy of all the groups on axial position. Draw cis-1-ethyl-4-methylcyclohexane in its lowest energy conformation 10 Part A Draw etyl-4-methylcyclohexane in its lowest energy conformation 1. The Chair Conformation - a closer look.
Another Article :

The boat conformation is again a puckered structure that allows tetrahedral bond angles. The single bond is active default GH. I take it you are talking about substituted and disubstituted cyclohexanes. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. The lower energy chair conformation contains two axial methyl groups. Is lowest energy conformation most stable. Which Is The Lowest Energy Conformation Of Butane Youtube.

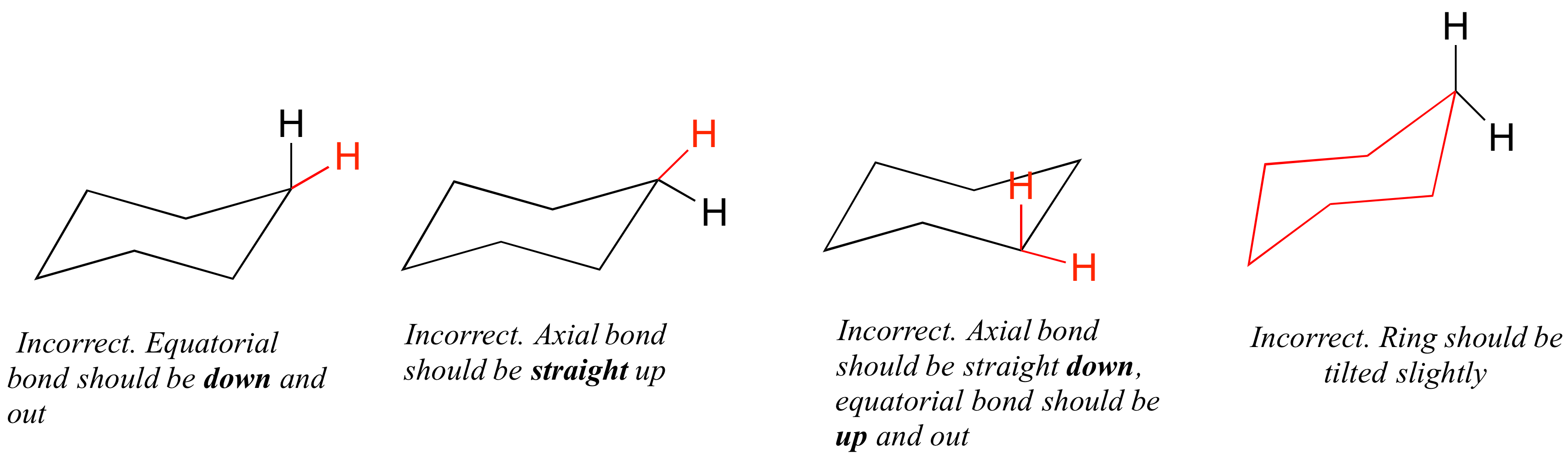
The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. For cis-13-dimethylcyclohexane the lowest energy chair conformation implies that the methyl groups of 1 and 3 carbons of the cyclohexane ring must occupy the equatorial positions. The staggered conformation of ethane is. Another conformation which is important in any conformational analysis is the transition state or maximum energy conformation on the rotational pathFor cyclohexane this is the so-called half-chair conformation in which now 5 carbons are co-planar and only one is puckered out of the plane. Rank these two low energy conformers as more or less stable. The lower energy chair conformation contains two axial methyl groups. Cyclohexane Conformational Analysis.
A second much less stable conformer is the boat conformation. Draw the following chair in the most stable conformation. Chair twist boat boat half-chair. For each chair conformer add the energy of all the groups on axial position. Draw cis-1-ethyl-3-methylcyclohexane in its lowest energy conformation. A second much less stable conformer is the boat conformation. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
Run a molecular dynamics simulation in order to get a sense of the flexibility of the cyclohexane ring. For cis-13-dimethylcyclohexane the lowest energy chair conformation implies that the methyl groups of 1 and 3 carbons of the cyclohexane ring must occupy the equatorial positions. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. Run a molecular dynamics simulation in order to get a sense of the flexibility of the cyclohexane ring. Calculating Flip Energy. Lowest energy form is the most important. 3 2 Conformations Of Cyclic Organic Molecules Chemistry Libretexts.
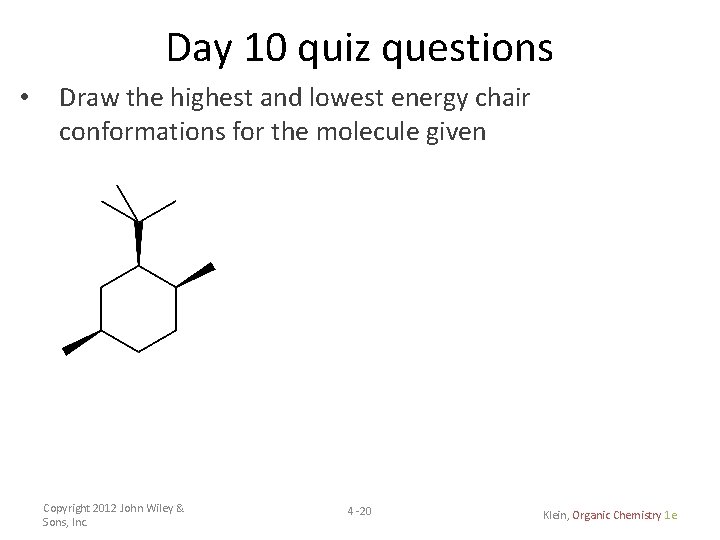
The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strainThis means that the chair conformation is the structure. The boat conformation suffers from torsional strain making it less stable higher in energy than the chair. Draw cis-1-ethyl-4-methylcyclohexane in its lowest energy conformation 10 Part A Draw etyl-4-methylcyclohexane in its lowest energy conformation 1. The chair conformation is the most stable conformation of cyclohexane. Draw the second chair conformation ring-flip-check this post if not sure. The other conformations are known as the twist and boat forms. Day 2 Quiz Questions Draw The Lewis Structure.
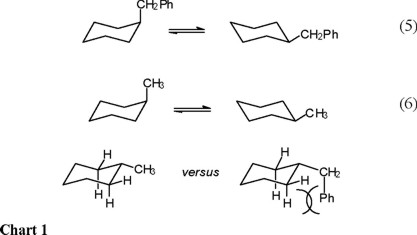
The chair conformation is the most stable conformation of cyclohexane. A cyclohexane is a six-membered saturated cyclic ring with a chemical formula eqrm C_6H_12 eq. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. Experts are tested by Chegg as specialists in their subject area. And models will always be permitted in examinations. Draw cis-1-ethyl-4-methylcyclohexane in its lowest energy conformation 10 Part A Draw etyl-4-methylcyclohexane in its lowest energy conformation 1. Enthalpic And Entropic Contributions To The Conformational Free Energy Differences In Monosubstituted Cyclohexanes.
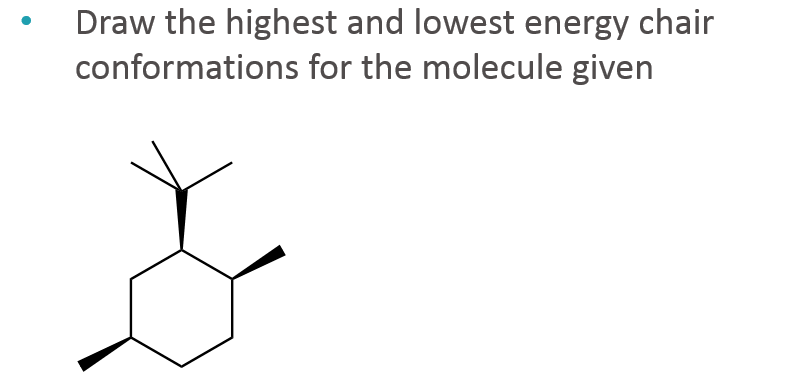
If they occupy the axial positions then it will be the highest energy chair conformation because of. A The two chair conformations are equal in energy. On careful examination of a chair conformation of cyclohexane we find that the twelve hydrogens are not structurally equivalent. The single bond is active default GH. A cyclohexane is a six-membered saturated cyclic ring with a chemical formula eqrm C_6H_12 eq. Explaining how A-Values are related to cyclohexane flip energy. Solved Draw The Highest And Lowest Energy Chair Chegg Com.
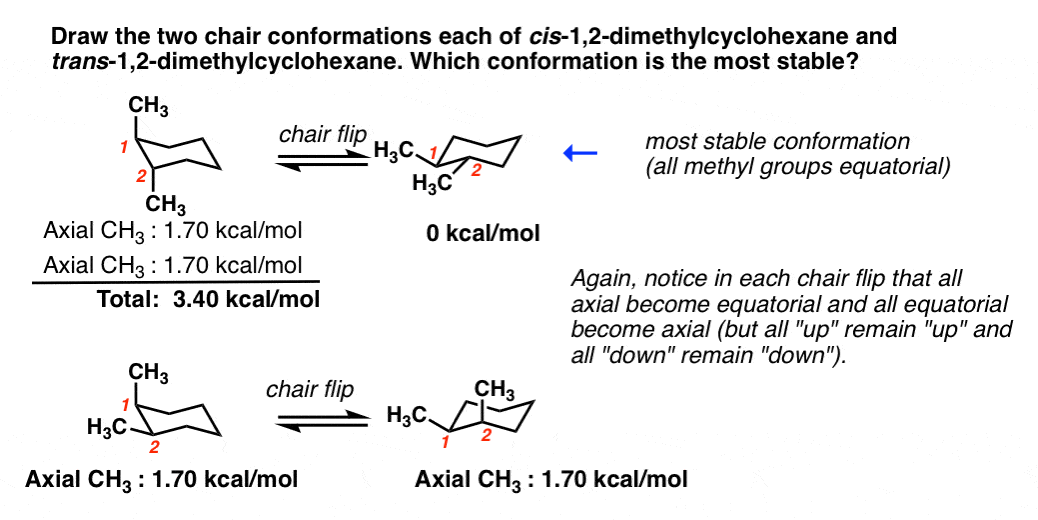
The twist boat has a lower energy than the boat. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. The chair conformation that you have drawn 4 C 1 is likely to be the most stable one as it minimizes the number of heavy axial groupsTo determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1If the number of heavy axial groups becomes smaller when the conformation. The boat conformation suffers from torsional strain making it less stable higher in energy than the chair. Chair twist boat boat half-chair. The other conformations are known as the twist and boat forms. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.

There is no substitute for the use of models. The structure above is the most stable structure of trans-1-ethyl-2-isopropylcyclohexane hence it has the lowest energy. A second much less stable conformer is the boat conformation. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. The boat conformation is again a puckered structure that allows tetrahedral bond angles. Draw The Lowest Energy Chair Conformation Clutch Prep.
Show transcribed image text Expert Answer. The chair conformation is the most stable conformation of cyclohexane. The twist boat has a lower energy than the boat. Experts are tested by Chegg as specialists in their subject area. I take it you are talking about substituted and disubstituted cyclohexanes. Draw the lowest energy chair conformer for each structure A and B. 1 Draw The Lowest Energy Energy Chair Conformation Chegg Com.
![]()
Which of the following is the lowest energy chair conformation of the following cyclohexane. And now the stabilities. For each chair conformer add the energy of all the groups on axial position. Chair twist boat boat half-chair. The chair conformation that you have drawn 4 C 1 is likely to be the most stable one as it minimizes the number of heavy axial groupsTo determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1If the number of heavy axial groups becomes smaller when the conformation. I take it you are talking about substituted and disubstituted cyclohexanes. Draw Cis 1 Ethyl 3 Methylcyclohexane In Its Lowest Energy Conformation Study Com.

So the relative stabilities are. If they occupy the axial positions then it will be the highest energy chair conformation because of. A The two chair conformations are equal in energy. The structure above is the most stable structure of trans-1-ethyl-2-isopropylcyclohexane hence it has the lowest energy. In the boat conformation two of the substituents those on the bow and the stern if you will are brought close enough to each other to cause. Number the ring and draw any chair conformation of the compound. Solution Draw The Lowest Energy Chair Co Organic Chem.

The other conformations are known as the twist and boat forms. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. The chair conformation is the most stable conformation of cyclohexane. In order to go from the chair conformation to a twist-boat conformation or the other chair conformation bond angles have to be changed leading to a high-energy half-chair conformation. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. Choose a chair from the Templates toolbar at the bottom. Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps.
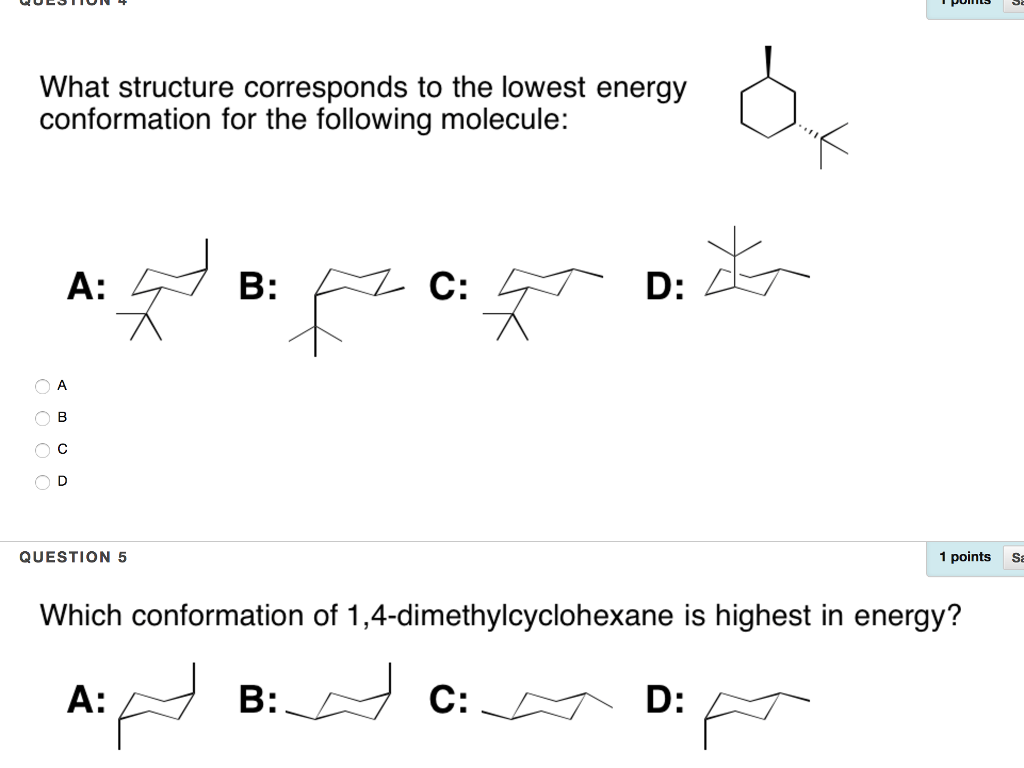
Draw the second chair conformation ring-flip-check this post if not sure. Rank these two low energy conformers as more or less stable. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. Notice that half of the hydrogen atoms have a different chemical environment. A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. Another conformation which is important in any conformational analysis is the transition state or maximum energy conformation on the rotational pathFor cyclohexane this is the so-called half-chair conformation in which now 5 carbons are co-planar and only one is puckered out of the plane. Solved What Structure Corresponds To The Lowest Energy A Chegg Com.
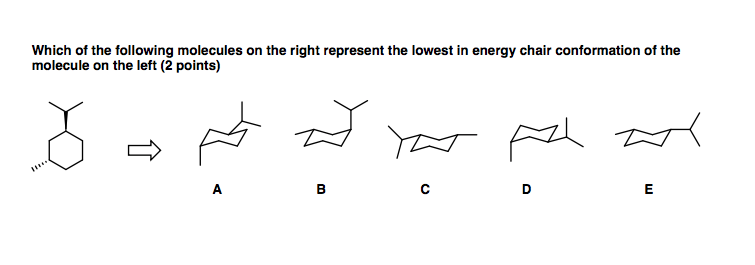
In the first conformer we have two chlorines in axial positions so the total steric strain is. Draw the second chair conformation ring-flip-check this post if not sure. For cis-13-dimethylcyclohexane the lowest energy chair conformation implies that the methyl groups of 1 and 3 carbons of the cyclohexane ring must occupy the equatorial positions. A cyclohexane is a six-membered saturated cyclic ring with a chemical formula eqrm C_6H_12 eq. Notice that half of the hydrogen atoms have a different chemical environment. A The two chair conformations are equal in energy. Solved Which Of The Following Molecules On The Right Chegg Com.